What elements form interstitial alloys?
Answers
Elements that form interstitial alloys are, carbon, nitrogen, boron, hydrogen, oxygen, sulfur, phosphorus.
Interstitial alloys are formed when smaller atoms or ions occupy the interstitial spaces (gaps) within the crystal lattice of a metal. These smaller atoms or ions are typically non-metallic and have a smaller atomic size compared to the host metal.
The formation of interstitial alloys is commonly observed in transition metals, as they have relatively open crystal structures that allow for the incorporation of smaller atoms. Some of the elements that form interstitial alloys include:
Carbon (C): Carbon can form interstitial alloys with iron to create steels, which are widely used in various applications due to their enhanced mechanical properties.
Nitrogen (N): Nitrogen can form interstitial alloys with titanium, resulting in materials with improved strength and hardness.
Boron (B): Boron is often used to form interstitial alloys with metals such as cobalt and nickel, leading to improved wear resistance and high-temperature stability.
Hydrogen (H): Hydrogen can form interstitial alloys with metals like palladium and titanium, resulting in materials with unique properties such as enhanced hydrogen storage capacity.
Interstitial compounds with other non-metals: Elements such as oxygen, sulfur, and phosphorus can also form interstitial compounds with metals, contributing to changes in the mechanical, electrical, or magnetic properties of the resulting alloy.
It's important to note that the formation of interstitial alloys depends on factors such as the crystal structure of the metal, the size and electronic compatibility of the interstitial element, and the conditions under which the alloy is synthesized.
Learn more about alloys at: https://brainly.com/question/31309979
#SPJ11
Related Questions
What types of reactions take place in hot packs and cold packs? What evidence shows that
both hot packs and cold packs experience chemical reactions? Drag and drop the text into
the correct boxes.

Answers
Answer:
reaction to cold pack: change in temperature
Evidence of a chemical reaction etc is change in composition
Explanation: composition is atoms changeing and etc, which is a chemical reacton i think. Change in temperture is alsoa chemical reaction but cold packs
Answer:
Cold pack: absorbs heat (endothermic reaction)
Hot pack: releases heat ( exothermic reaction)
Explanation:
when a cold pack/hot pack is shaken, water mixes with the chemicals in the outer layer and reacts to release or absorb heat.
What is the name of the molecule shown below?

Answers
Answer:
B) Methanoic Acid
Explanation:
You have an object with a mass of 14 g and a volume of 20 mL. Calculate the density and identify which material you have from the list.
Answers
Answer:
0.7 g/ml. Whichever material matches that closest is the right one.
what is the molar concentration of lithium ions in a 0.550 m li3po4 solution?what is the molar concentration of lithium ions in a 0.550 m li3po4 solution?2.20 m5.00 m1.65 m0.550 m0.183 m
Answers
The molar concentration of lithium ions in the Li3PO4 solution is 1.65 M.
To determine the molar concentration of lithium ions in a Li3PO4 solution, we need to consider the ratio of lithium ions to Li3PO4 in the compound.
In Li3PO4, there are three lithium ions (Li+) for every one formula unit of Li3PO4. Therefore, the molar concentration of lithium ions will be three times the molar concentration of Li3PO4.
Given that the molar concentration of Li3PO4 is 0.550 M, the molar concentration of lithium ions will be:
0.550 M × 3 = 1.65 M
Learn more about concentration here
https://brainly.com/question/13872928
#SPJ11
which point on the potential energy diagram corresponds to the species shown to the right for the electrophilic nitration of benzene with hno3/h2so4
Answers
Point (c) C on the potential energy diagram corresponds to the species shown to the right for the electrophilic nitration of benzene with hno3/h2so4.
There have been many other observed substitution reactions involving benzene; the five reactions that have proven to be the most beneficial are described here (chlorination and bromination are the most common halogenation reactions). The term "Electrophilic Aromatic Substitution" is usually used to refer to these reactions since the chemicals and conditions that are used in them are electrophilic. In order to carry out the first stage of the substitution reaction, the catalysts and co-reagents are used to produce the powerful electrophilic species that are required. The particular electrophile that was hypothesized to have a role in each distinct type of reaction is presented in the column to the right.
Point C on the potential energy diagram corresponds to the species shown to the right for the electrophilic nitration of benzene with hno3/h2so4.
The complete question is attached.
You can also learn about potential energy from the following question:
https://brainly.com/question/24284560
#SPJ4

if the mass number of an atom is 23 and the number of neutrons in the nucleus of an atom is 12, then the atomic number is:
12
23
11
35
Answers
in gas chromatography, a liquid mixture is injected and converted into a mixture of gases that are separated based on their boiling points, the separation occurs in the
Answers
A liquid mixture is injected and converted into a mixture of gases that are separated based on their boiling points in gas chromatography, the separation occurs in the chromatographic column.
What is gas chromatography?Gas chromatography is a method used to analyze and separate volatile organic compounds. A liquid mixture is injected into a chromatographic column that contains a stationary phase and a carrier gas is introduced, which then flows through the column. The sample components get separated as they move at different rates through the column and interact with the stationary phase. This separation is based on their boiling points and affinity to the stationary phase.
Gas chromatography has a wide range of applications including environmental testing, forensic analysis, pharmaceuticals, and petrochemicals. The method is also used in the food and beverage industry to analyze and identify flavor compounds in food products.
Learn more about gas chromatography: https://brainly.com/question/6870684
#SPJ11
Write copper (I) chlorate in a chemical formula.
Answers
The chemical formula for copper (I) chlorate is CuClO3. This is a compound of copper in the oxidation state +1 and chlorate, which is an ion with the formula ClO3^- and a charge of -1
Copper(I) chlorate is formed when copper(I) ions combine with chlorate ions. The copper(I) ion has a +1 charge, while the chlorate ion has a -1 charge. To balance the charges, one copper(I) ion combines with one chlorate ion. The chlorate ion is represented by the chemical formula ClO3 with a -1 charge. The copper(I) ion is represented by Cu with a +1 charge. Therefore, the chemical formula for copper(I) chlorate is CuClO3.
To know more about chemical formulas : brainly.com/question/32018188
#SPJ11
A scientist recorded the following observations about a rock sample in her journal.
The rock is porous.
The rock has multiple layers.
The rock contains a fossil.
What type of rock did the scientist collect?
igneous , igneous , ,
sedimentary , sedimentary , ,
metamorphic , metamorphic , ,
obsidian
Answers
Answer:
Sedimentary rocks
Explanation:
Sedimentary rocks are types of rock that are formed by the accumulation or deposition of mineral or organic particles at Earth's surface, followed by cementation.
The process of deep sediment burial causes sediment to get compressed and cemented resulting in sedimentary rock. Here the recorded observations of the scientist is about sedimentary rocks. The correct option is B.
What are sedimentary rocks?The rocks which are formed by the deposition and subsequent cementation of the material at the surface of the earth within the bodies of water can be defined as the sedimentary rocks. They are of three types. They are organic, clastic and chemical sedimentary rocks.
Sedimentary rocks are generated by the accumulation of the existing rocks or fragments of extinct organisms on the earth surface. They are formed from the pre-existing rocks or pieces of once living organisms.
The common sedimentary rocks are sandstone, limestone and shale. Biologic sedimentary rocks are formed when large number of living things die. Chert is an example for this.
Thus the correct option is B.
To know more about sedimentary rocks, visit;
https://brainly.com/question/10709497
#SPJ2
does an oxygen atom form two covalent bonds?
Answers
Answer:
yes
Explanation:
Two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell.
The National Stock Number (NSN) test search is used to locate test reports and Special Packaging Instructions (SP) on which of the following?
Answers
The NSN test search is used to locate test reports that provide comprehensive information about the testing and evaluation of specific products or items.
The National Stock Number (NSN) test search is used to locate test reports on various products or items. Test reports provide detailed information about the testing and evaluation of a particular product's performance, quality, safety, or compliance with specific standards or requirements. These reports are often conducted by independent testing laboratories or organizations to assess the characteristics and capabilities of the product.
The NSN test search allows users to search for test reports based on the NSN, which is a unique identification number assigned to each item in the federal supply system. By inputting the NSN into the search, individuals or organizations can access relevant test reports associated with that specific item or product.
The test reports obtained through the NSN test search can be valuable for various purposes, such as quality assurance, product development, procurement decisions, or regulatory compliance. They provide important insights into the performance and reliability of the item being tested, allowing users to make informed decisions based on objective evaluation and testing data.
In summary, the NSN test search is used to locate test reports that provide comprehensive information about the testing and evaluation of specific products or items.
Learn more about testing from below link
https://brainly.com/question/27794277
#SPJ11
pls help asap you can!

Answers
The frequency of the color light, given that it has a wavelength of 5.0×10⁻⁷ m is 6.0×10¹⁴ Hertz (last option)
How do i determine the frequency of the color light?First, we shall list out the given parameters from the question. This is shown below:
Wavelength of color light (λ) = 5.0×10⁻⁷ mSpeed of color light (v) = 3×10⁸ m/sFrequency of color light (f) =?Speed, wavelength and frequency of wave are related by the following formula:
Velocity (v) = wavelength (λ) × frequency (f)
Inputting the given parameters, we can obtain the frequency as shown below:
3×10⁸ = 5.0×10⁻⁷ × frequency
Divide both sides by 5.0×10⁻⁷
Frequency = 3×10⁸ / 5.0×10⁻⁷
= 6.0×10¹⁴ Hertz
Thus, we can conclude that the frequency of the color light is 6.0×10¹⁴ Hertz (last option)
Learn more about frequency:
https://brainly.com/question/23060871
#SPJ1
an atom of vanadium (z = 23) in its ground state has _____ valence electrons.
Answers
An atom of vanadium (Z = 23) in its ground state has 5 valence electrons.
Valence electrons are the electrons in the outermost energy level of an atom. In the case of vanadium, it has an electron configuration of [Ar] 4s^2 3d^3. The outermost energy level is the 4s orbital, which contains 2 electrons, and the 3d orbital, which contains 3 electrons. The total number of electrons in the outermost energy level, therefore, is 5, making them the valence electrons. Valence electrons play a crucial role in determining the chemical properties and reactivity of an element. They are involved in bonding with other atoms and determining the element's ability to form compounds.
To learn more about electrons click here: brainly.com/question/12001116 #SPJ11
the vapor pressure of water is 92.5 torr at 50.0 celsius. what is the vapor pressure of a solution that has 35 grams of nacl dissolved in 115 grams of water at this temperature?
Answers
The vapour pressure of a solution containing 35 grammes of NaCl dissolved in 115 grammes of water at 50.0°C is approximately 84.5 torr.
The vapour pressure of the resultant solution is lower than the vapour pressure of the pure solvent when a solute is added to a solvent. The amount of this phenomenon, referred to as the reduction of vapour pressure, depends on the concentration of the solute in the solution.
Raoult's law, which says that the vapour pressure of a solution is equal to the vapour pressure of the pure solvent multiplied by the mole fraction of the solvent, may be used to determine the vapour pressure of a solution that includes a certain quantity of solute:
Psolution = Xsolvent * P°solvent
Xsolvent is the mole fraction of the solvent, P°solvent is the vapour pressure of the pure solvent, and Psolution is the vapour pressure of the solution.
We must first determine the mole fraction of water in the solution before we can apply this equation to the presented situation. Since the masses of water and NaCl are known, we can convert the masses to moles by using the molar masses of each substance:
Moles of NaCl are equal to 35 g / 58.44 g/mol, or 0.599 mol.
115 g divided by 18.02 g/mol yields 6.386 moles of water.
Therefore, the total moles in the solution are equal to the moles of NaCl plus the moles of water, which equals 0.599 mol plus 6.386 mol, or 6.985 mol.
Therefore, Xwater = moles of water / total moles = 6.386 mol / 6.985 mol 0.915 is the mole percentage of water in the solution.
Now we can determine the solution's vapour pressure using Raoult's law:
Psolution is calculated as follows: Psolution = Xwater * P°water, where P°water is the vapour pressure of pure water at 50.0°C, which is given to be 92.5 torr.
Psolution is equal to 0.915 x 92.5 84.5 torr.
As a result, the solution's vapour pressure at 50.0 °C and 35 g of NaCl dissolving in 115 g of water is around 84.5 torr.
For such more question on Pressure
https://brainly.com/question/24719118
#SPJ4
Use the change of base rule to find the logarithm to four decimal places. log 143.2 O 0.2213 O 4.5186 2.2593 O
0.4771
Answers
Using the change of base rule to find the logarithm to four decimal places. the correct answer is 11.4235.
To find the logarithm of 143.2 using the change of base rule, we can choose any base we prefer. Let's use base 10 and natural logarithm (base e) for this calculation.
First, we'll use the change of base formula, which states that log(base b) x = log(base c) x / log(base c) b. In this case, we'll calculate log(base 10) 143.2.
We'll use the natural logarithm (ln) as our intermediary step. The natural logarithm of 143.2 can be calculated as ln(143.2).
Using a calculator, we find that ln(143.2) is approximately 4.9628.
Next, we need to calculate log(base 10) e, which is the logarithm of e with base 10. Using a calculator, we find log(base 10) e is approximately 0.4343.
Finally, we apply the change of base formula:
log(base 10) 143.2 ≈ ln(143.2) / log(base 10) e
≈ 4.9628 / 0.4343
≈ 11.4235
Rounding to four decimal places, the logarithm of 143.2 using base 10 is approximately 11.4235.
Learn more about logarithm here :-
https://brainly.com/question/30226560
#SPJ11
what is the predominant sweetener used in formulating beverages?
Answers
The predominant sweetener used in formulating beverages is high-fructose corn syrup (HFCS).
High-fructose corn syrup (HFCS) is a common sweetener widely used in the formulation of beverages. It is derived from corn starch and consists of varying proportions of glucose and fructose. The most common types of HFCS used in beverages are HFCS-55 (which contains approximately 55% fructose and 45% glucose) and HFCS-42 (which contains approximately 42% fructose and 58% glucose).
HFCS is favored in the beverage industry due to its sweetness, affordability, and ease of use as a liquid sweetener. It dissolves easily in water and can be added to a wide range of beverage formulations, including carbonated drinks, fruit juices, sports drinks, and flavored waters.
While HFCS is a prevalent sweetener, it is important to note that other sweeteners such as sucrose (table sugar), artificial sweeteners (e.g., aspartame, sucralose), and natural sweeteners (e.g., stevia, monk fruit extract) are also used in beverage formulations, depending on factors such as taste preferences, cost, and desired product characteristics.
learn more about high-fructose corn syrup here:
https://brainly.com/question/32821004
#SPJ11
What is the coefficients for HgO __ Hg + O2
Answers
Answer:
the coefficients for HgO2 Hg+O2
Explanation:
the coefficient is 2
Hg= mercury element 80 aka quick silver
O2=oxygen can not have less than 2 atoms or it will be unstable so it forms a bond between the 2 oxygen atoms
why is the average number of fish species not a whole number?
Answers
Answer:
Because in order to find the average, you need to divide by the number of sites tested. Sometimes that doesn't divide evenly.
Explanation:
what is the formula for vanadium oxide
Answers
Vanadium(V) oxide is the inorganic compound with the formula V₂O₅. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent.
formula:V2O5
pls mark as brainliest
Answer:
is it vanadium v oxide or just vanadium oxide
Explanation:
a 5.325 g sample of methyl benzoate is found to contain 3.758 g of carbon, 0.316 g of hydrogen, and 1.251 g of oxygen, what is the empirical formula of this substance
Answers
The empirical formula is C₄H₄O.
To find the empirical formula of methyl benzoate, we'll follow these steps:
Step 1: Convert mass to moles
Carbon: 3.758 g / 12.01 g/mol (molar mass of C) = 0.313 moles
Hydrogen: 0.316 g / 1.008 g/mol (molar mass of H) = 0.313 moles
Oxygen: 1.251 g / 16.00 g/mol (molar mass of O) = 0.078 moles
Step 2: Divide by the smallest mole value (0.078 moles)
Carbon: 0.313 moles / 0.078 = 4.01
Hydrogen: 0.313 moles / 0.078 = 4.01
Oxygen: 0.078 moles / 0.078 = 1.00
Step 3: Round to the nearest whole number
Carbon: 4
Hydrogen: 4
Oxygen: 1
Step 4: Combine the whole numbers
The empirical formula is C₄H₄O.
To know more about empirical formula here:
https://brainly.com/question/14044066#
#SPJ11
The pH of a solution in which OH negative concentration equals six. 9×10 to the -10 molarity
Answers
Answer:
The pH of a solution in which the concentration of hydroxide ions is 6.9 x 10^-10 M is approximately 4.84.
Explanation:
The pH of a solution can be calculated from the concentration of hydroxide ions (OH-) using the relationship between pH, pOH, and the ion product constant for water (Kw). The pOH of a solution is defined as the negative logarithm (base 10) of the hydroxide ion concentration: pOH = -log[OH-]. The pH and pOH of a solution are related by the equation pH + pOH = 14. The ion product constant for water at 25°C is Kw = [H+][OH-] = 1.0 x 10^-14.
In this case, the concentration of hydroxide ions is given as [OH-] = 6.9 x 10^-10 M. We can use this value to calculate the pOH of the solution:
pOH = -log[OH-] = -log(6.9 x 10^-10) = 9.16
Then, we can use the relationship between pH and pOH to calculate the pH of the solution:
pH + pOH = 14
pH + 9.16 = 14
pH = 14 - 9.16
pH = 4.84
Therefore, the pH of a solution in which the concentration of hydroxide ions is 6.9 x 10^-10 M is approximately 4.84.
Answer:
pH = 4.84
Explanation:
To find the pH of a solution, we can use the formula:
pOH = -log[OH-]
Given:
[OH-] = 6.9 × 10^-10 M
First, let's calculate the pOH of the solution:
pOH = -log(6.9 × 10^-10)
pOH ≈ -(-9.16)
pOH ≈ 9.16
Now, to find the pH, we can use the relation:
pH + pOH = 14
pH = 14 - pOH
pH = 14 - 9.16
pH ≈ 4.84
Therefore, the pH of the solution is approximately 4.84.
How many of each subatomic particle are found in Tc-99?
Answers
Answer:
43 protons and 99 neutrons.
Explanation:
PLEASE HELP ME!!!
THE CHEM QUESTION IS...
Calculate the amounts of heat required for 25.0 g of water to change from 10°C to 30°C
Answers
Answer:
1045
Answer: 1045 J of energy was released on cooling the down the water from 20 °C to 10 °C.
Answer:
..............=2100
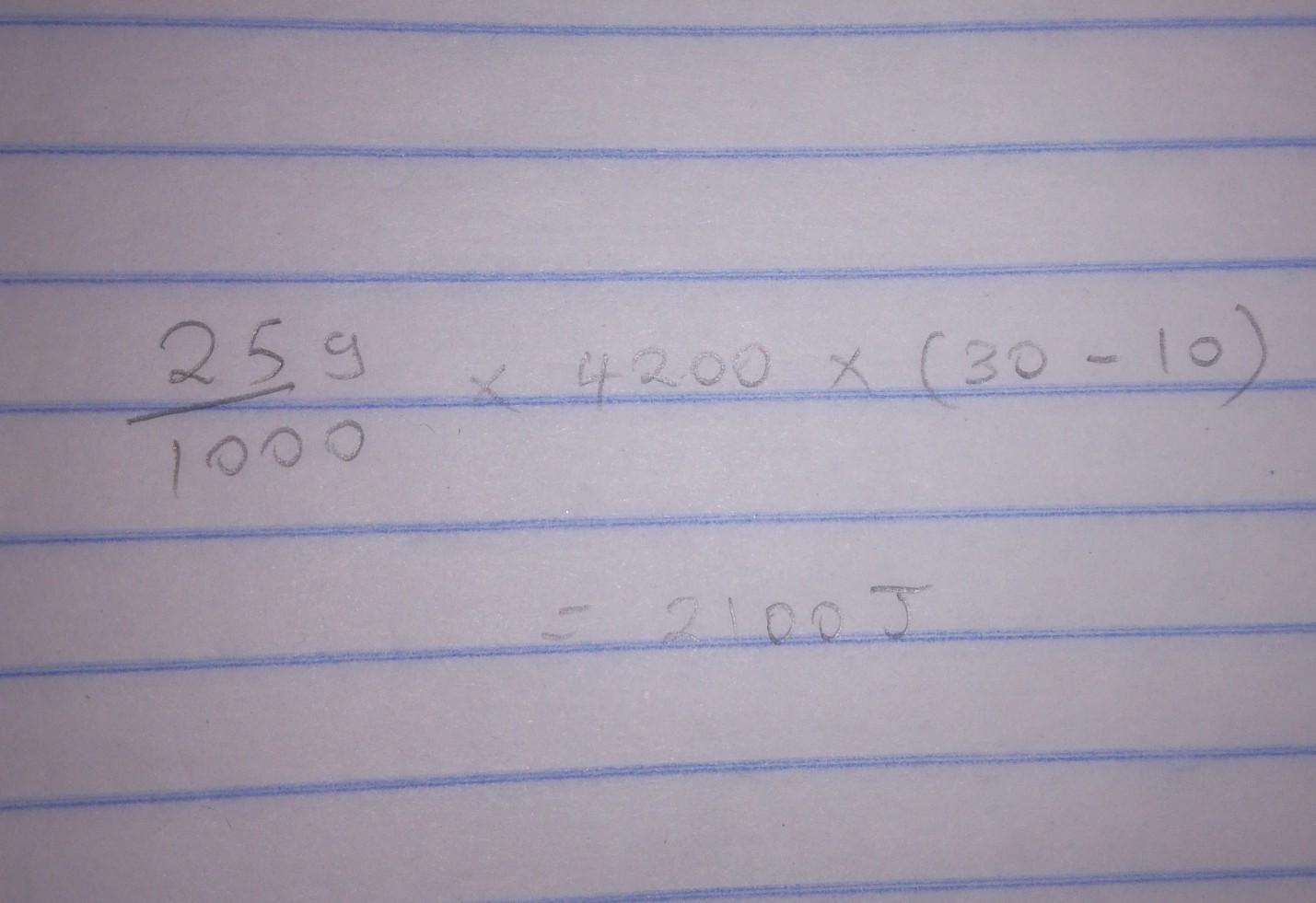
1) 120KM/H A M/S Y CM/S
2) 80KM/H A M/S Y CM /S
3) 75 M/S A KM/H y M/MIN
4) 35KM/H A M/S ; M/MIN ; CM/ S
nesecito ayuda pliss
Answers
2)22.22m/s and 2222cm/s
3)270 km/h and 4500m/min
4) 9.72 m/s and 583.3 m/min and 972.2 cm/s
No one believed him b/c he had no
Answers
3. Which of the rocks from the table would be classified as metamorphic rocks?
A.
Gneiss and slate, only
B.
Granite, only
C.
Limestone and basalt, only
D.
Coal, only
Answers
What is the acronym is a reminder of the most common types of hazards caused by electricity?.
Answers
According to the acronym be safe included in OSHA's Electrocution hazards handbook, an electrical hazard is a situation that occurs at work that exposes employees to the following risks are burns, the most frequent injury brought on by shock.
What is an electrical hazard ?Electric shock and burns from contact with live components are the two primary risks of working with electricity. harm from arcing exposure, fire from improper electrical installations, or both
An electrical danger can be found before it poses a problem by inspecting tools and equipment before use. Regular inspections should be performed on all tools to look for fractures, frayed cord lines, damaged insulation, broken ground pins, and loose parts.
Thus, the Occupational Safety and Health Administration when describing the dangers that electricity may pose to construction workers.
To learn more about electrical hazard, follow the link;
https://brainly.com/question/14374685
#SPJ1
A chemist adds a chemical to pure water and there is a 100-fold increase in the concentration of hydrogen ions. What is the best approximation of the new ph value?.
Answers
If a chemist adds a chemical to pure water and there is a 100-fold increase in the concentration of hydrogen ions then the best approximate value of new pH is 5.
When only pure water is there.
H₂O ⇌ H⁺ + OH⁻
pKw = pH + pOH
14 = pH + pH ( This is because the value of pH and pOH is same in water and we need to consider pH here.)
14 = 2pH
pH= 14/2 = 7
So, pH of pure water is 7.
Concentration of hydrogen ions in pure water can be calculated using the equation
pH = -log[H⁺]
7 = -log[H⁺]
[H+] = 10⁻⁷ M
So, the concentration of hydrogen ions in pure water is 10⁻⁷ M.
Now, the chemist added a chemical to this pure water such that there is 100- fold increase in concentration of hydrogen ions.
So, the new concentration of hydrogen ions is calculated as shown below.
[H⁺] = 100 ×10⁻⁷ M
= 10⁻⁵ M
The new concentration of hydrogen ions is 10⁻⁵ M.
Now, the new pH can be calculated using the formula
pH = -log[H⁺]
= -log[10⁻⁵]
= -×-5log[10]
= 5×1
= 5
So, the new pH value = 5.
To know more about pH here
https://brainly.com/question/2288405?referre
#SPJ4
Which best explains the relationship between evaporation and temperature?
A liquid evaporates slower at lower temperatures because the molecules are more spread apart and are not pushed as easily from the liquid’s surface.
A liquid evaporates faster at lower temperatures because the attractions are decreased and more particles can escape the surface of the liquid.
A liquid evaporates slower at higher temperatures because the vapor pressure of the liquid is higher, so fewer molecules can escape the surface.
A liquid evaporates faster at higher temperatures because more particles have a higher speed and can overcome attractions in the liquid.
Answers
Answer:
A liquid evaporated slow at higher temperature bz the vapour pressure of the is higher so few molecule can escape the surface
Answer:
The correct answer is D. A liquid evaporates faster at higher temperatures because more particles have a higher speed and can overcome attractions in the liquid.
Explanation:
I just got it right on Edge.
Hope this helped!
Brainliest would be greatly appreciated :D
Select the correct answer. Two charged objects, A and B, exert an electric force on each other. What happens if the distance between them is increased? A. The charge on B decreases. B. The charge on B increases. C. The electric force between them decreases. D. The electric force between them increases.
Answers
Answer:
The electric force between them decreases
Explanation:
The force between two charged particle is given by :
\(F=\dfrac{kq_1q_2}{r^2}\)
Where
r is the distance between charges
If the distance between the charges is increased, the electric force gets decreased as there is an inverse relation between force and distance.
Hence, the correct option is (c) "The electric force between them decreases"