What happens energetically when chemical bonds are formed?.
Answers
When chemical bonds are formed, energy is usually released. When atoms join, they form a molecule by a chemical bond, which is a mutual attraction between the nuclei and the valence electrons of different atoms.
When atoms unite to form a molecule, the bond energy becomes negative due to the release of energy. If we take, for example, the combination of hydrogen and chlorine gas to form hydrogen chloride, it is an exothermic reaction because energy is released during the formation of H-Cl.
For example, energy is liberated as a result of this process, resulting in a release of energy that is either absorbed or emitted in the form of heat or light. This energy is required to overcome the electrostatic attraction between the nuclei and the electrons so that a molecule can be formed. When chemical bonds are formed, energy is released, and when chemical bonds are broken, energy is absorbed.
You can learn more about chemical bonds at: brainly.com/question/30387060
#SPJ11
Related Questions
3. Which of the following is NOT an assumption of the
kinetic-molecular theory?
a. Collisions between gas particles are elastic.
b. All the gas particles in a sample have the samel
velocity
c. A gas particle is not significantly attracted or
repelled by other gas particles.
d. All gases at a given temperature have the same aver-
age kinetic energy.
Answers
Calcium has an atomic number of 20. An atom of calcium has ____ electrons in its first energy level and ____ electrons in its second energy level.
Answers
give the si base unit of each of these quantities. enter the abbreviation rather than the name of the unit.
Answers
The SI base unit for length is meter (m), for mass is kilogram (kg), for time is second (s), for electric current is ampere (A), for temperature is kelvin (K), for amount of substance is mole (mol), and for luminous intensity is candela (cd).
Length: The SI base unit for length is meter (m), which is the standard unit for measuring distance or size. Mass: The SI base unit for mass is kilogram (kg), which is the standard unit for measuring the amount of matter in an object. Time: The SI base unit for time is second (s), which is the standard unit for measuring the duration or interval between events.
Electric current: The SI base unit for electric current is ampere (A), which is the standard unit for measuring the flow of electric charge. Temperature: The SI base unit for temperature is kelvin (K), which is the standard unit for measuring the average kinetic energy of particles in a substance. Amount of substance: The SI base unit for amount of substance is mole (mol), which is the standard unit for measuring the quantity of particles (atoms, molecules, etc.) in a substance. Luminous intensity: The SI base unit for luminous intensity is candela (cd), which is the standard unit for measuring the brightness of light emitted by a source.
To know more about mass visit:
https://brainly.com/question/11954533
#SPJ11
london dispersion forces . (select all that apply.) multiple select question. are found within nonpolar molecules are stronger for larger molecules than smaller ones are intermolecular attractions are not affected when a liquid evaporates are much weaker than the covalent bonds within the hydrocarbon molecule
Answers
London dispersion forces are: a)Found within nonpolar molecules b)Stronger for larger molecules than smaller ones d) Much weaker than the covalent bonds within the hydrocarbon molecule. So, options a, b, d applied.
The London dispersion force considered as the weakest intermolecular force. The London dispersion force is considered as a temporary attractive force that results when the electrons present in two adjacent atoms occupy the positions that make the atoms to form the temporary dipoles. This force is sometimes called as induced dipole-induced dipole attraction
These forces are defined for the molecule. These type of forces behaves positively when temperature changes. When we increase the temperature ,in that case molecules starts making distance from each other due to that intermolecular force of attraction gets weaker.
Learn more about London dispersion forces at:
https://brainly.com/question/4301021
#SPJ4
(Complete question) is:
London dispersion forces . (select all that apply.) multiple select question. are
a)Found within nonpolar molecules
b)Stronger for larger molecules than smaller ones
c)Intermolecular attractions are not affected when a liquid evaporates
d)Much weaker than the covalent bonds within the hydrocarbon molecule
4. How much energy will it take to raise the temperature of 75.0 g of water from 20.0 °C to 55.0 °C?
Specific heat of water = 4.184 J/(g°C)
A. 63 J
B. 630 J
C. 2630 J
D. 1.1 x 10¹ J
Answers
Answer:
1.1 x 10⁴ J
Explanation:
To calculate eth energy needed, you need to use the following equation:
Q = mcΔT
In this equation,
-----> Q = energy/heat (J)
-----> m = mass (g)
-----> c = specific heat (4.184 J/g°C)
-----> ΔT = change in temperature (°C)
You can plug the given values into the equation and solve.
Q = mcΔT
Q = (75.0 g)(4.184 J/g°C)(55.0 °C - 20.0 °C)
Q = (75.0 g)(4.184 J/g°C)(35.0)
Q = 11,000 J
Q = 1.1 x 10⁴ J
10. How many grams of potassium are needed for potassium to react
with 4.20 liters of chlorine gas to produce potassium chloride at
19.0°C and 27.0 atms?
2K (s) + Cl₂ (g)-2KCI (aq)
O
O
4.73 grams
92.5 grams
369 grams
2840 grams
Answers
m(K) is equal to 1126 g, or 1.13 kg, or 28.8 mol times 39.10 g/mol. As a result, none of the suggested solutions are the correct one.
How is km to g converted?As a result, to convert from kg to g, you must double the amount before kg by 1000. You can learn to divide the number preceding the unit by 1000 by using the examples of kilogrammes and grammes.
How do you change g from scientific notation to kg?One kilogramme is equal to 1,000 grammes; the word "kilo" stands for 1,000. The prefix kilo is represented by the sign k, and the unit of mass known as the gramme is represented by the symbol g.kg. So ½ kg of bananas is the same as 500 g of bananas, or 5 × 102 g of bananas.
To know more about mol visit:-
https://brainly.com/question/24191779
#SPJ1
What kind of system is the coffee cup calorimeter considered?
Answers
A coffee cup calorimeter is a type of basic calorimeter. A calorimeter measures the heat transfer between a system and its surroundings.
The coffee cup calorimeter is a basic calorimeter made comprised of a Styrofoam cup, a thermometer, and a stirrer. It is used to calculate the amount of heat emitted or absorbed during a chemical reaction or physical change, such as salt dissolving in water.
The reaction occurs in the cup of a coffee cup calorimeter, and heat is absorbed or released by the cup and its contents. The quantity of heat involved in the reaction can be calculated by monitoring the temperature change in the cup.
While not as precise as more advanced calorimeters, such as bomb calorimeters, the coffee cup calorimeter is a helpful tool for measuring the heat involved in many common chemical processes, and it is reasonably simple and affordable to use.
To Learn More About reaction click
https://brainly.com/question/28984750
#SPJ4
How do the ideas of electrolytes and IV fluids relate?
Answers
Answer:
Electrolytes, particularly sodium, help the body maintain normal fluid levels in the fluid compartments because the amount of fluid a compartment contains depends on the amount (concentration) of electrolytes in it. If the electrolyte concentration is high, fluid moves into that compartment (a process called osmosis).
Explanation:
Answer:
Electrolytes are minerals in your body that have an electric charge. They are in your blood, urine, tissues, and other body fluids. Electrolytes are important because they help
Balance the amount of water in your body
Balance your body's acid/base (pH) level
Move nutrients into your cells
Move wastes out of your cells
Make sure that your nerves, muscles, the heart, and the brain work the way they should
Sodium, calcium, potassium, chloride, phosphate, and magnesium are all electrolytes. You get them from the foods you eat and the fluids you drink.
The levels of electrolytes in your body can become too low or too high. This can happen when the amount of water in your body changes. The amount of water that you take in should equal the amount you lose. If something upsets this balance, you may have too little water (dehydration) or too much water (overhydration). Some medicines, vomiting, diarrhea, sweating, and liver or kidney problems can all upset your water balance.
Treatment helps you to manage the imbalance. It also involves identifying and treating what caused the imbalance.
hope it's help you plz mark as brain listWhat is the planet's albedo? group of answer choices its ability to reflect light its ability to produce carbon dioxide its ability to absorb light its ability to product stratospheric ozone
Answers
The planet's albedo has ability to reflect light.
The planetary albedo would be the percentage of incoming solar radiation that Earth scatters back into space. The processes that control the quantity, distribution, and fluctuation of this reflected energy are crucial to the Earth's energy balance and have a significant impact on both climate including climate change.
Temperatures rise as a result of carbon dioxide, prolonging the growing season as well as raising the humidity. Each of these elements has stimulated some further plant growth. But hotter weather also stresses plants. Plants require more water to live in an extended, warmer growing season.
Therefore, the planet's albedo has ability to reflect light.
To know more about planet's albedo
https://brainly.com/question/7138899
#SPJ4
If anyone is taking Integrated Physics and Chemistry (2014) on sos can u pls help me w something !! Thanks
Answers
I am so sorry i cant help but good luck
concentrated sodium hydroxde (naoh) must be treated with caution because it is choose... . proper protective equipment includes choose... and choose... .
Answers
Concentrated sodium hydroxide (NaOH) must be treated with caution because it is a highly corrosive and caustic substance. Proper protective equipment includes chemical-resistant gloves and safety goggles.
Handling concentrated sodium hydroxide requires strict safety measures due to its potential to cause severe burns and damage to the skin, eyes, and respiratory system. In addition to chemical-resistant gloves and safety goggles, other protective equipment such as a lab coat, closed-toe shoes, and even a face shield can be used to minimize the risk of exposure. In case of accidental contact, it is crucial to have an eyewash station and safety shower nearby to quickly rinse off any NaOH that comes into contact with the skin or eyes.
Furthermore, it is essential to work in a well-ventilated area to prevent the inhalation of harmful fumes, and proper storage guidelines must be followed. Sodium hydroxide should be stored in a tightly sealed, labeled container, away from any acidic or flammable materials. Lastly, it is important to be knowledgeable about emergency procedures and first-aid measures to handle any potential accidents or incidents involving concentrated NaOH.
Know more about Concentrated sodium hydroxide here:
https://brainly.com/question/28612681
#SPJ11
19. What type of atmosphere do the inner planets have?
Answers
Answer: nitrogen and carbon dioxide
Explanation: I hope this helps :)
Atomic spectroscopy - refer to attached picture for question. Good and clear answer, will get brainliest.

Answers
The detector would show various colors to show the elements in the mixture from the flame.
What is spectroscopy?The whole idea of the use of spectroscopy is that we are able to study the elements by the use of light. We know that light can be able to reveal the properties or the components that we have in a system. In this case, we have a lamp that is the light source (lamp) and then we have a flame where we have to put in the sample to be analyzed and then we have the detector that shows the spectrum of the substance that is read out.
We know that we would have a pure spectrum when we have sin gle lines in the spectrum. If we have a spectrum that is jumbled up and there are so many lines in the spectrum then we could come up with the idea that there is a mixture of substance as we would see a combination of colors in the spectrum of the element.
Learn more about spectrum:https://brainly.com/question/6836691
#SPJ1
Mrs. Keep burns a walnut under a beaker of water. The beaker contains 100 g of water which warms from 25oC to 30oC. Assuming that all the heat from the burning walnut goes into the water and none of the heat is lost to the air or the beaker, how many calories are in the walnut?
a 2100 calories
b 10,500 calories
c not enough information is given
d 500 calories
Answers
The amount of heat gained by the water is 500 calories. Thus, option D is correct.
Given:
Mass of water (m) = 100 g
Change in temperature (ΔT) = 30°C - 25°C = 5°C
The specific heat capacity of water (c) is approximately 1 calorie/gram°C.
Now, the amount of heat gained by the water,
Q = mcΔT
Where:
Q is the heat gained or lost by the substance
m is the mass of the substance
c is the specific heat capacity of the substance
ΔT is the change in temperature
Plugging in the values into the formula:
Q = 100 × 1 × 5
Q = 500 calories
Therefore, the amount of heat gained by the water is 500 calories.
Learn more about heat, here:
https://brainly.com/question/31608647
#SPJ1
What is the definition of momentum?
A- The amount of matter in an object.
B-Mass in motion.
C-Speed with direction.
D- The resistance to change motion
Answers
Answer:
B. Mass in motion. Is the definition of momentum
An alkene adds hydrogen in the presence of a catalyst to give 3,4-dimethylhexane. Ozonolysis of the alkene followed by treatment with zinc and acetic acid gives a single organic product. The structure of the alkene is: CH3 CH3CH
Answers
The structure of alkene is given below in the image.
Alkenes are described as both branched or unbranched hydrocarbons that possess at least one carbon-carbon double bond (CC) and feature a popular system of CnH2n [1].
Alkanes are organic compounds that encompass single-bonded carbon and hydrogen atoms. The formulation for Alkanes is CnH2n+2, subdivided into 3 companies – chain alkanes, cycloalkanes, and branched alkanes.
The geometry around each carbon atom is based totally on a trigonal planar shape, due to the fact, that each carbon has three electrons around it. This must make the angle of each bond one hundred twenty.
Learn more about Alkanes here: https://brainly.com/question/17040500
#SPJ4
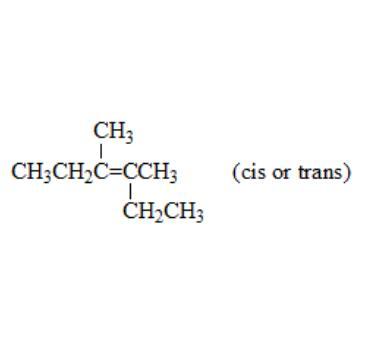
What is the concentration of H+ at pH 4 in moldm-3 ?
Answers
Answer:
The pH of a solution is a measure of its concentration of hydrogen ions. The higher the concentration of H + ions in an acidic solution, the lower the pH. A pH of 1 represents a hydrogen ion concentration of 0.1 mol/dm 3.
...
pH and hydrogen ion concentration.
Concentration pH
0.001 mol/dm 3 3
0.0001 mol/dm 3 4
Explanation:
Answer:
0.0001
That the answer your welcom
please help i will mark brainliest which helps bring up youre rank

Answers
Answer:
push or pull
Explanation:
What bonding pattern do you observe that you could use to predict whether a compound will be gas at standard temperature and pressure?
Answers
Answer:What bonding pattern do you observe that you could use to predict whether a compound will be gas at standard temperature and pressure?
The element in group 14, period 3, of the periodic table is classified as a.
Answers
Explanation:
Silicon (symbol Si) is a group 14 metalloid.
Drag each tile to the correct image.
Match each alkane name with its structure.
octane
decane
propane
butane
heptane
CHE
IGH
CHE
Reset
Next

Answers
Answer:
The first one is Propane
The second one is HEPTANE
The third one is octane
The 4th is butane
the 5th is decane
The structures have been named according to IUPAC as \(\rm C_3H_8\) Propane, \(\rm C_7H_{16}\) Heptane, \(\rm C_8H_{18}\) Octane, \(\rm C_4H_{10}\) Butane, and \(\rm C_{10}H_{22}\) Decane.
The images has been the representation of the ball and stick structure of the compounds. The central balls have been the representation of the carbon atom , with small balls attached to the sticks have been the representation of the hydrogen attached.
The following structures has been given as:
The structure has 3 carbon atoms with the presence of 8 hydrogen. The molecular formula has been \(\rm C_3H_8\). It has been the structure of propane.The structure has 7 carbon and 16 hydrogen. The structure has been the representation of heptane with molecular formula \(\rm C_7H_{16}\).The structure has molecular formula \(\rm C_8H_{18}\) with 8 carbon and 18 hydrogen. It has been named Octane, according to IUPAC.The structure with 4 carbon and 10 hydrogen with molecular formula \(\rm C_4H_{10}\) has been named according to IUPAC as butane.The structure with molecular formula \(\rm C_{10}H_{22}\) has presence of 10 carbon and 22 hydrogen. It has been named as Decane.The structures have been named according to IUPAC as \(\rm C_3H_8\) Propane, \(\rm C_7H_{16}\) Heptane, \(\rm C_8H_{18}\) Octane, \(\rm C_4H_{10}\) Butane, and \(\rm C_{10}H_{22}\) Decane.
For more information about structure of hydrocarbons, refer to the link:
https://brainly.com/question/8049265
Chymotrypsin has an α-helix that contains 2.5 turns. Approximately how many amino acids are involved in this helix?A. 20.25B. 9C. 13.5D. 3.75E. 2.5
Answers
Chymotrypsin has an α-helix that contains 2.5 turns. Approximately 9 amino acids are involved in this helix.
What is chymotrypsin?
The pancreas produces chymotrypsin. Chymotrypsinogen is its precursor. By cleaving peptide bonds in locations Arg15–Ile16, trypsin activates chymotrypsinogen and generates –chymotrypsin. The "oxyanion hole" and the hydrophobic "S1 pocket" are the results of the interaction between the aminic group (-NH3+) of the Ile16 residue and the side chain of Asp194. Additionally, chymotrypsin causes its own activation by cleaving at positions 14, 15, 146, and 148 to create -chymotrypsin, which is both more active and stable than -chymotrypsin. A three-polypeptide molecule with disulfide bonds connecting them makes up the final product.To know more about chymotrypsin, click the link given below:
https://brainly.com/question/13638833
#SPJ1
A hydrogen atom makes a downward transition from the n=19 state to the n=5 state, Find the wavelength of the emitted photon. 2.45μm 2.94μm 1.47μμm 1.96μμm
Answers
The wavelength of the emitted photon is approximately 2.44 μm.
The wavelength of the emitted photon can be determined using the Rydberg formula as follow
\(\frac{1}{\lambda}=R_H\left(\frac{1}{n_1^2}-\frac{1}{n_2^2}\right)\)
where: lambda is the wavelength of the emitted photon,
\(R_H=1.0974\times10^7\text{m}^{-1} ,$n_1=19 n_2=5 :\frac{1}\)
\({\lambda}=R_H\left(\frac{1}{19^2}-\frac{1}{5^2}\right) \frac{1}\\\\\\{lambda}=1.0974\times10^7\text{m}^{-1}\left(\frac{1}{361}-\frac{1}{25}\right) \frac{1}\\{\lambda}=1.0974\times10^7\text{m}^{-1}\left(0.002709-0.04\right) \frac{1}\\{\lambda}=1.0974\times10^7\text{m}^{-1}\times(-0.037291) \frac{1}{\lambda}=-409446.34\text{m}^{-1} \lambda=-\frac{1}{409446.34\text{m}^{-1}}=2.44\times10^{-6}\text{m}\)
Therefore, the wavelength of the emitted photon is approximately 2.44 μm.
Rounded to two decimal places, this value is equal to 2.45 μm. Thus, the correct option is A) 2.45μm.
Learn more about wavelenght with the given link,
https://brainly.com/question/10750459
#SPJ11
What if a small amount of air leaked back into the flask through the tightened screw clamp as the flask assembly was cooling? would your calculated value for the molar mass of air be too high, too low, or would there be no effect? explain
Answers
The measured volume of hydrogen gas will be too high.
The volume of hydrogen is measured by collecting the gas over water. The volume of the gas is measured as the volume of water displaced by the gas in an inverted container.
When air leaks into the graduated cylinder, more volume of water is displaced hence a higher volume of hydrogen gas is measured.The calculated molar volume of hydrogen will be too high as a result of this error
The gas being recorded during the experiment would not only include hydrogen gas, but the air that leaked into the eudiometer tube as well. This leads to the increase in the volume of hydrogen gas which will be too high.
Since the volume of hydrogen is too high, therefore the calculated molar volume of hydrogen would also be too high.
To know more about volume, refer: brainly.com/question/10609459
#SPJ4
What is the MOLECULAR formula for a compound that has the empirical formula S3O2 and a molecular mass of 144. 3 grams?
Answers
The molecular formula for the compound with the empirical formula S3O2 and a molecular mass of 144.3 g is S6O4.
The given empirical formula for a compound is S3O2 and the molecular mass of that compound is 144.3 g. Let's find the molecular formula for the given compound using the given information.The empirical formula mass of the given compound can be calculated by adding the atomic masses of each of its atoms together based on their empirical formula as follows:S3O2 ⇒ (3 × atomic mass of S) + (2 × atomic mass of O)⇒ (3 × 32.1) + (2 × 16)⇒ 96.3 + 32⇒ 128.3 g/molThe molecular formula of a compound is the integer multiple of its empirical formula. To calculate the molecular formula of the given compound, we need to find the ratio between its empirical formula mass and molecular mass as follows:Molecular formula mass / Empirical formula mass = n (an integer multiple)Molecular formula mass / 128.3 g/mol = n (an integer)Molecular formula mass = n × 128.3 g/molThe molecular mass of the given compound is 144.3 g/mol.Therefore, n = Molecular formula mass / Empirical formula massMolecular formula mass = n × Empirical formula mass = n × 128.3 g/mol = (144.3 g/mol)/nLet's check if n = 2 is correct. When n = 2, we get the following molecular formula mass:Molecular formula mass = n × Empirical formula mass= 2 × 128.3 g/mol= 256.6 g/molThe molecular formula of the given compound is S6O4 when n = 2. The empirical formula mass of the compound is 128.3 g/mol. Therefore, the molecular formula for the compound with the empirical formula S3O2 and a molecular mass of 144.3 g is S6O4.
learn more about molecular
https://brainly.com/question/10778879
#SPJ11
If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in each syringe? (Hint: Choose one volume and temperature pair from your data table to use in your ideal gas law calculation.)

Answers
Answer:
A: 2.525 x 10-4 mol
B: 2.583 x 10-4 mol
Explanation:
Part A:
Data Given:
. Temperature of water (H2O) = 21.3°C
Convert Temperature to Kelvin
T = °C + 273
T = 21.3 + 273 = 294.3 K
volume of (H2O) gaseous state = 5.1 mL
Convert mL to liter
1000 mL = 1L
5.1 ml = 5.1/1000 = 0.0051 L
Pressure = 1.2 atm
. no. of moles = ?
Solution
no. of moles can be calculated by using ideal gas formula
PV = nRT
Rearrange the equation for no. of moles
n=PV/RT......... (1)
where
P = pressure
V = Volume
T= Temperature
n = Number of moles
R = ideal gas constant
where
R = 0.08206 L.atm/ mol. K
Now put the value in formula (1) to calculate no. of moles of
n = 1.2 atm x 0.0051 L / 0.08206 L.atm.mol-1. K-1 x 294.3 K
n = 0.0061 atm.L / 24.162 L.atm.mol-1
n = 2.525 x 10-4 mol
no. of moles of gas (H2O) = 2.525 x 10-4 mol
Part B:
Data Given:
Temperature of water (H2) = 21.3°C
Convert Temperature to Kelvin
T = "C + 273
T= 21.3 + 273 = 294.3 K
volume of (H2) gas = 5.2 mL
Convert mL to liter
1000 mL = 1 L
5.2 ml = 5.2/1000 = 0.0052 L
Pressure = 1.2 atm
. no. of moles = ?
Solution
no. of moles can be calculated by using ideal gas formula
PV = nRT
Rearrange the equation for no. of moles
n= PV / RT......... (1)
where
P = pressure
V = Volume
T= Temperature
n = Number of moles
R = ideal gas constant
where
R = 0.08206 L.atm/mol. K
Now put the value in formula (1) to calculate no. of moles of
n = 1.2 atm x 0.0052 L/0.08206 L.atm.mol-1. K-1 x 294.3 K
n = 0.0062 atm.L/ 24.162 L.atm.mol-1
n = 2.583 x 10-4 mol
I
no. of moles of gas (H2) = 2.583 x 10-4 mol
1.Which group of numbers can divide 54 with no remainder?
A. 1, 2, 5, 7, 12,54
B. 1, 2, 6,9, 27,54
C. 2, 4, 6, 8, 26, 54
D. 1, 2, 7, 8, 29,54
Answers
Answer:
The answer is B. 1,2,6,9,27,54
Explanation:
If the temperature of a fixed quantity of gas decreases by a factor of two and the pressure decreases by a factor of four, then?
Answers
If the temperature of a fixed quantity of gas decreases by a factor of two and the pressure decreases by a factor of four, then the ratio of pressure (P₂) to temperature (T₂) remains constant.
If the temperature of a fixed quantity of gas decreases by a factor of two and the pressure decreases by a factor of four, we can determine the relationship between temperature, pressure, and volume using the combined gas law.
The combined gas law states:
P₁V₁ / T₁ = P₂V₂ / T₂
Let's assume that the volume (V) remains constant in this scenario, as the problem states a fixed quantity of gas.
Temperature factor (T₁/T₂) = 2 (temperature decreases by a factor of two)
Pressure factor (P₁/P₂) = 4 (pressure decreases by a factor of four)
Put these values into the combined gas law:
P₁V₁ / T₁ = P₂V₂ / T₂
Since V₁ = V₂ (constant volume), we can cancel out the volume terms:
P₁ / T₁ = P₂ / T₂
Now, let's substitute the given factors into the equation:
4 / 2 = P₂ / T₂
2 = P₂ / T₂
From this equation, we can conclude that the ratio of pressure (P₂) to temperature (T₂) remains constant. In other words, if the temperature of a fixed quantity of gas decreases by a factor of two and the pressure decreases by a factor of four, their ratio (P₂/T₂) will remain the same.
To know more about temperature here
https://brainly.com/question/7510619
#SPJ4
2500m into kilometer
Answers
1 meter = 1000 km
2500 meter = 2500/1000 km
= 2.5 km
What is the speed of a cheetah that runs 30 miles in 0.5 hours?
a
Answers
Answer:
60 mph (miles per hour)
Explanation:
0.5 hours is 1/2 of an hour, so to get the number of miles for a whole hour you multiply the miles ran by 2.
30 times 2 is 60.
S = 30/0.5 = 60mi/ hr