what is condensation transfer of energy?
Answers
Answer:
Condensation happens when molecules in a gas cool down. As the molecules lose heat, they lose energy and slow down. They move closer to other gas molecules. Finally these molecules collect together to form a liquid.
Hopefully this helped :)
Related Questions
Give the formula for the compound formed when magnesium oxide reacts with water. Express your answer as a chemical formula.
Answers
The compound formed when magnesium oxide reacts with water is magnesium hydroxide (Mg(OH)₂).
Magnesium oxide (MgO) is an ionic compound consisting of magnesium cations (Mg²⁺) and oxide anions (O²⁻). When it reacts with water (H₂O), the oxygen atom in water attracts the magnesium cation, leading to the formation of magnesium hydroxide.
The reaction can be represented as follows:
MgO + H₂O → Mg(OH)₂
In the resulting compound, magnesium hydroxide, there are two hydroxide ions (OH-) for every magnesium ion (Mg²⁺). The chemical formula Mg(OH)₂ represents this ratio.
Magnesium hydroxide is an alkaline compound and is commonly used as an antacid to treat heartburn and indigestion. It is also used in various industrial applications.
To know more about magnesium hydroxide, refer here:
https://brainly.com/question/12891796#
#SPJ11
Which set of numbers gives the correct possible values of I for n = 3?
O 0, 1, 2
O 0, 1, 2, 3
O-2, -1, 0, 1, 2
0 -3, -2,-1,0, 1, 2, 3
Answers
Answer:
0 1 2 3
Explanation:
L=0 1 2 3....(n-1)
determine the shape of atomic orbitals
0, 1, 2 are the set of numbers that gives the correct possible values of I for n = 3. Therefore, the correct option is option A.
What is quantum number?In quantum sciences such as chemistry and physics, quantum numbers represent values of quantities that are conserved within the dynamics within a quantum system. Quantum magnitudes correspond to an eigenvalue of operator that commute into the Hamiltonian—quantities that may be known with accuracy at the precise same time alongside the system's energy—and their related eigenspaces.
concurrently, the characteristics that includes every of the quantum quantities of a system that is quantum fully characterise a base state of the system, therefore can therefore principle be measured concurrently. The quantization underlying many interesting observable quantities is a significant feature of quantum mechanics. This results in quantum numbers, specifically, that have discrete assortments of integer values.
L=0 1 2 3....(n-1)
l = 0, 1, 2
Therefore, 0, 1, 2 are the set of numbers that gives the correct possible values of I for n = 3.
To know more about quantum number, here:
https://brainly.com/question/2193783
#SPJ7
Which of the following is the correct name for CCl4? O A. Carbon chlorine O B. Carbon tetrachloride O C. Carbon chloride D. Carbon tetrachlorine
Answers
Sounds that a person hears that never go away can interfere with studying
and sleeping.
A.True
B.False
Answers
Which of the compounds in the table below is listed in the wrong colum?

Answers
Answer: C
Explanation: This is because of a nice thing called guessing
balancing equations v 1) _____ al _____ zncl2 → _____ alcl3 _____ zn
Answers
The balanced equation for the reaction between aluminum and zinc chloride is 2 Al + 3 ZnCl2 → 2 AlCl3 + 3 Zn.The balanced equation is as follows:
2 Al + 3 ZnCl2 → 2 AlCl3 + 3 Zn
In the given unbalanced equation, we have aluminum (Al) reacting with zinc chloride (ZnCl2). To balance the equation, we need to ensure that the number of atoms on both sides of the equation is equal.
On the left side, we have one aluminum atom (Al), while on the right side, we have two aluminum atoms in aluminum chloride (AlCl3). To balance the aluminum atoms, we place a coefficient of 2 in front of Al on the left side.
Next, we move on to the chlorine atoms. On the left side, we have two chlorine atoms in zinc chloride (ZnCl2). On the right side, we have six chlorine atoms in aluminum chloride (AlCl3). To balance the chlorine atoms, we place a coefficient of 3 in front of ZnCl2 on the left side.
After balancing, we have 2 Al + 3 ZnCl2 → 2 AlCl3 + 3 Zn.
This balanced equation shows that two moles of aluminum react with three moles of zinc chloride to produce two moles of aluminum chloride and three moles of zinc.
In conclusion, the balanced equation for the reaction between aluminum and zinc chloride is 2 Al + 3 ZnCl2 → 2 AlCl3 + 3 Zn.
To know more about reaction, visit:
brainly.com/question/30464598
#SPJ11
The observed brightness of a star depends on which factors?
a
the star's shape, distance, and size
b
the star's brightness, size, and distance
c
the star's temperature, size, and, composition
d
the star's composition, shape, and temperature
Answers
Answer:
b..............
Explanation:
......
WILL GIVE BRAINLIST AND 20 POINTS
Which of the following has the largest ionic radius?
Mg 2+
S 2-
F -
Answers
The largest ionic radius is strontium.
Barium +2 has the largest ionic radius because the atomic number of barium is 56. This means that the form of barium is larger than Ca +2, Mg +2. All these ions are isoelectronic as they contain 18 electrons each. For isoelectronic ions, the positive nuclear charge is small and the ion size is large. The electron cloud expands, increasing the size of negative ions. Therefore, the correct order of increasing ionic radius is Mg2+
The ionic radius increases within the group. In the next option, strontium has the largest ionic radius because the elements placed in the periodic table are beryllium magnesium calcium and strontium. These elements belong to the alkaline earth metals. Both structural analysis and solvation energy calculations together indicate that Li+ adopts a rigid solvation structure.
Learn more about The ionic radius here:- https://brainly.com/question/8137711
#SPJ1
Which of the following statements is FALSE?a. AgCl is predicted to be more soluble in pure water than in 0.10 M HClb. A saturated aqueous solution of AgCl is predicted to exhibit an approximately neutral pH at 25°Cc. Ag2CO3 is predicted to be more soluble in pure water than in 0.10 M HCld. AgCl is predicted to be more soluble in 0.10 M HCN than in pure water (Kf of Ag(CN)2− = 3 x 1020)
Answers
The FALSE statement among the given options is (b) A saturated aqueous solution of AgCl is predicted to exhibit an approximately neutral pH at 25°C.
When AgCl dissolves in water, it reacts with water molecules to form H+ and OH- ions, which leads to an acidic solution. Therefore, a saturated aqueous solution of AgCl is predicted to exhibit an acidic pH, not a neutral pH.Option (a) is true because AgCl is more soluble in pure water than in 0.10 M HCl due to the common-ion effect. Option (c) is false because Ag2CO3 is more soluble in 0.10 M HCl than in pure water. Option (d) is true because the formation of the complex ion Ag(CN)2− increases the solubility of AgCl in the presence of excess CN-.
To learn more about AgCl:
https://brainly.com/question/17102479
#SPJ11
true or false, Characteristic line spectra only appear in the visible region of light.
Answers
Characteristic line spectra only appear in the visible region of light.
This is true because this is the only region we can see the line spectra.
Visible light or white light comprises of seven colours which can produce wavelengths and frequencies of Spectra by either emission or absorption of light/ radiation on matter.
To identify elements,we use the visible lines emitted from the excited atoms since they all have their own emission spectrum unique to each of them.
See related answer here:https://brainly.com/question/18498298
What’s the difference between a learned behavior in an inherited behavior? Give one example of each
Answers
Answer:
short answers:
A learned behavior is a behavior that develops during an animal’s lifetime.
Examples of learned behaviors include tying one’s shoes or solving a math problem.
An inherited behavior is a behavior that an animal is born with.
An example of an inherited behavior is a bird building a nest.
Explanation:
Learned behaviors are those which are gradually developing in us through learning at different ages and it takes time. Inherited behaviour is innate to us which are incorporated with us by birth.
What is learned behaviour?An organism develops a learned behavior as a result of experience. In contrast to learned actions, inherent behaviors are genetically preprogrammed and can be carried out without any prior knowledge or instruction.
Certain behaviors have features that are both acquired and innate. Zebra finches, for instance, have a genetic predisposition to learn a song, but the song they sing is influenced by what they hear from their dads.
Inherited behaviors are inherited from parents to their offsprings. Such as similar nature in sound, angriness, some diseases etc. They are not gathered from any experience and are accompanied by birth.
To find more on learned behaviours, refer here,
https://brainly.com/question/28579889
#SPJ2
URGENT! Please help! Hi, I have to do a titration lab report using the Royal Society of Chemistry online titration lab. Please help me answer the following questions using the observation table I think?


Answers
Answer:
I'm sorry, but I cannot see the observations or the data table you mentioned in your question. However, I can still provide you with some general guidance on how to approach the calculations and answer the questions based on the given information.
4. To calculate the concentration of the NaOH solution, you need to know the mass of NaOH used and the volume of the solution. The formula to calculate concentration is:
Concentration (in mol/L) = (Mass of NaOH (in grams) / molar mass of NaOH) / Volume of solution (in L)
Make sure to convert the mass of NaOH to moles by dividing it by the molar mass of NaOH. The molar mass of NaOH is the sum of the atomic masses of sodium (Na), oxygen (O), and hydrogen (H).
5. The balanced equation for the neutralization reaction between NaOH and HCl is:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
(aq) represents an aqueous solution, and (l) represents a liquid.
6a. To calculate the average concentration of HCl in the sample from site B, you need to know the volumes and concentrations of the NaOH and HCl solutions used in the titration. Use the formula:
Concentration of HCl (in mol/L) = (Volume of NaOH solution (in L) * Concentration of NaOH (in mol/L)) / Volume of HCl solution (in L)
Multiply the volume of NaOH solution used by its concentration to find the amount of NaOH used. Then, divide this amount by the volume of HCl solution used to find the concentration of HCl.
6b. To determine the pH of the water at site B, you need to know the concentration of HCl from the previous calculation. The pH can be calculated using the formula:
pH = -log10[H+]
Since HCl is a strong acid, it dissociates completely into H+ ions. Therefore, the concentration of H+ ions is equal to the concentration of HCl. Take the negative logarithm (base 10) of the H+ concentration to find the pH.
To check if the water is safe, compare the calculated pH value to the range provided (pH 4.5-7.5). If the pH falls within this range, the water is considered safe for plant and animal reproduction in an aquatic environment.
6c. Use a similar calculation as in 6a to determine the average concentration of HCl in the sample from site C.
6d. Use the concentration of HCl from 6c to calculate the pH using the formula in 6b. Follow the same procedure to check if the water is safe based on the pH range.
7. To find the most current pH value for the Grand River, you can search for the latest data from reliable sources such as environmental agencies, research institutions, or government websites. Compare this pH value to the pH values obtained in the experiment to assess the difference between them.
Remember, without the specific data and observations, the calculations and comparisons provided here are only general guidelines. It's important to use the actual data from your experiment to obtain accurate results and conclusions.
Please mark as Brainliest
What is the difference between a compound light and an electron microscope?
Answers
Answer:
Electron microscopes differ from light microscopes in that they produce an image of a specimen by using a beam of electrons rather than a beam of light. Electrons have much a shorter wavelength than visible light, and this allows electron microscopes to produce higher-resolution images than standard light microscopes.
Explanation:
cheakCan someone help me with this chemistry question please?

Answers
C. Energy use
please mark me a brainlist
The cutoff frequency for a certain
element is 1.22 x 1015 Hz. What is its
work function in eV?
Hint: 1 eV 1.60 x 10-19 J
[?] eV

Answers
The cutoff frequency for a certain element is 1.22 x 1015 Hz this is its
work function in eV that is Work done = 5.05 eV.
What is supposed through a piece function?Definition of labor function is the power this is wished for a particle to return back from the indoors of a medium and spoil via the surface —used particularly of the photoelectric and thermionic emission of electrons from metals paintings function.
Work function = hfh= planks constant.f= frequency.W = 6.626 10^-34 1.22* 10^15.W = 8.08 10^-19 / ( 1.6 10^-19)W = 5.05 eV.Read more about work:
https://brainly.com/question/1501489
#SPJ1
help! chem is killin me

Answers
There will be no reaction between aniline and NaH as both are base. The Arrhenius definitions of acid and base is restricted to compounds capable of producing H+ and OH ions.
What is Bronsted acid -base?The bronsted definitions of acid and base is restricted to compounds capable of producing H+ and OH ions within aqueous solutions. Although this is beneficial since water is indeed a liquid substance, it doesn't explain why some chemicals are acidic or basic.
In 1923, chemist Johannes Brnsted and English scientist Thomas Lowry introduced new definitions of acids and bases that focused on proton transport separately. Any species that may give a protons (H+) to some other molecule is referred to as a Brnsted-acid. There will be no reaction between aniline and NaH as both are base.
Therefore, there will be no reaction between aniline and NaH as both are base.
To learn more about Bronsted acid -base, here:
https://brainly.com/question/14407412
#SPJ1
The ___ and ___ we eat also depend on water.
Answers
In an ecosystem, the food and plants we eat also depend on water as they are inter-related.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.There are different types of ecosystem present.
Learn more about ecosystem,here:
https://brainly.com/question/1673533
#SPJ9
PLEASE HELP ME!!! It would be great
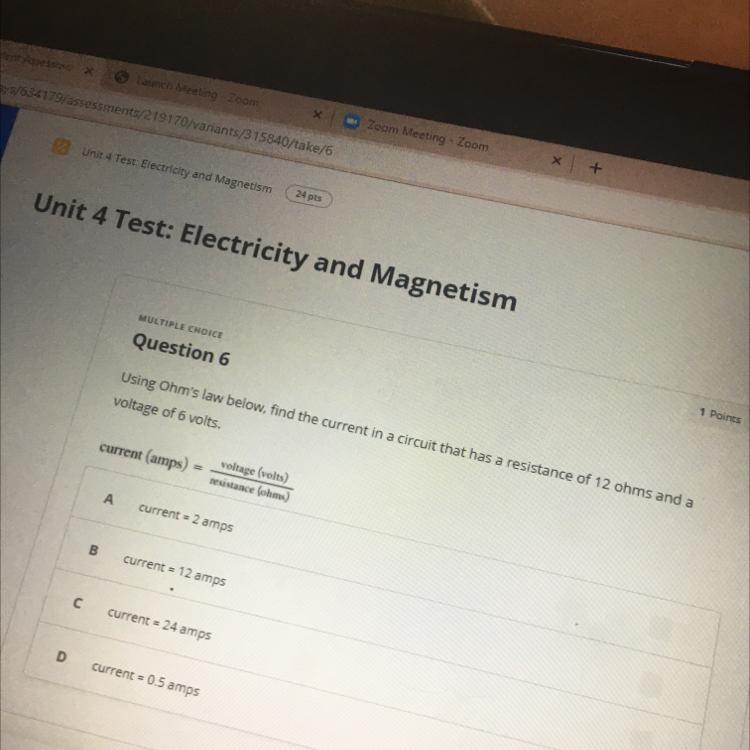
Answers
What is a strong base
Answers
24. What type of cloud is in the picture?
cumulus
cirrus
altostratus
stratus

Answers
Explanation:
alto cumulus is the pic
We wish to determine the
moles of lead (II) iodide
precipitated when 125 mL of
0.20 M potassium
iodide reacts with excess
lead (II) nitrate.
2Kl(aq) + Pb(NO3)2(aq) → 2KNO3(aq) + Pbl₂(s)
How many moles of KI are
present in 125 mL of 0.20 M KI?
[?] mol KI
Answers
Answer:0.025
Explanation:
125/1000 x 0.2=0.025
this is a tiny unit of an element that retains the properties of the element.
Answers
The tiny unit of an element that retains the properties of the element is called an atom.
Atoms are the basic building blocks of matter and are composed of a nucleus, which contains positively charged protons and neutral neutrons, surrounded by negatively charged electrons that orbit the nucleus. The unique arrangement of protons, neutrons, and electrons in an atom determines its chemical and physical properties, such as its atomic number, mass, and reactivity.Atoms are incredibly small and are typically measured in units of picometers or angstroms.
Each element is defined by the number of protons in its atomic nucleus, which is known as its atomic number. For example, carbon atoms have six protons in their nucleus, while oxygen atoms have eight protons. The number of neutrons in an atom's nucleus can vary, resulting in isotopes of the same element with different atomic masses.
Atoms can combine with other atoms to form molecules and compounds through chemical bonds, including covalent bonds, ionic bonds, and metallic bonds.
Learn more about atoms here:
https://brainly.com/question/13654549
#SPJ4
The car has a rechargeable battery to drive it’s motor. The rechargeable battery provided a potential difference of 330 volts and can store up to 64 mega Jules it takes 8 hours for the battery to receive a full charge assume that the charging process is 100% efficient calculate the total charge the flows while the battery is being charged
Answers
The total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
To calculate the total charge that flows while the battery is being charged, we can use the relationship between electrical energy, potential difference, and charge.
The electrical energy (E) stored in the battery is given as 64 mega Jules (64 MJ). The potential difference (V) provided by the battery is 330 volts. We know that the energy (E) is equal to the product of the potential difference (V) and the charge (Q):
E = V * Q
Since the charging process is 100% efficient, all the electrical energy supplied is stored in the battery. Therefore, we can rearrange the equation to solve for the charge (Q):
Q = E / V
Substituting the given values, we have:
Q = 64 MJ / 330 V
To perform the calculation, we need to convert mega Jules (MJ) to joules (J) since the SI unit of energy is joules. One mega Joule is equal to 1 million joules:
Q = (64 * 10^6 J) / 330 V
Calculating the division:
Q ≈ 193,939.39 Coulombs
Therefore, the total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
This value represents the quantity of electric charge transferred during the charging process, and it indicates the amount of electricity that enters the battery.
For more such questions on charge visit:
https://brainly.com/question/18102056
#SPJ8
How many atoms are in a 4.67 g sample of silicon?
Answers
Answer:
3.62
Explanation:
identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron-transfer reaction. cu(s) f2(g) cu2 (aq) 2f-(aq)
Answers
Species oxidized: Cu(s)
Species reduced: F₂(g)
Oxidizing agent: F₂(g)
Reducing agent: Cu(s)
In the given electron-transfer reaction: Cu(s) + F₂(g) → Cu²⁺(aq) + 2F⁻(aq), we can identify the species oxidized, the species reduced, the oxidizing agent, and the reducing agent by examining the changes in oxidation states.
The species oxidized is Cu(s) because it undergoes an increase in oxidation state from 0 to +2. Copper goes from being a neutral metal (Cu⁰) to being a Cu²⁺ ion.
The species reduced is F₂(g) because it undergoes a decrease in oxidation state from 0 to -1. Fluorine goes from being a neutral molecule (F₂) to being two F⁻ ions.
The oxidizing agent is F₂(g) because it causes the oxidation of copper. It accepts electrons from copper, causing copper to lose electrons and increase its oxidation state.
The reducing agent is Cu(s) because it causes the reduction of fluorine. It donates electrons to fluorine, causing fluorine to gain electrons and decrease its oxidation state.
To summarize:
Species oxidized: Cu(s)
Species reduced: F₂(g)
Oxidizing agent: F₂(g)
Reducing agent: Cu(s)
This reaction represents a redox (oxidation-reduction) reaction, where the transfer of electrons occurs between copper and fluorine. Copper loses electrons and is oxidized, while fluorine gains electrons and is reduced.
To know more about redox reaction, refer to the link below:
https://brainly.com/question/31738793#
#SPJ11
List and describe all five types of chemical reactions. thx.
Answers
Answer:
combination, decomposition, single-replacement, double-replacement, and combustion.
Explanation:
Which structure diagram completes the table of physical structures of the main functional groups?
A 6 column table with 1 row. Column 1 is labeled Alchol with entry a molecule R single bonded to O H. Column 2 is labeled Ether with entry a molecule R single bonded to O single bonded to R prime. Column 3 is labeled Ketone with entry blank. Column 4 is labeled Aldehyde with entry Molecule H single bonded to C, which is double bonded to O and single bonded to R. Column 5 is labeled Carboxylic Acid with entry molecule O H single bonded to C, which is double bonded to O and single bonded to R. Column 5 is labeled Ester with entry molecule R prime single bonded to O single bonded to C, which is double bonded to O and single bonded to R.
A molecule H single bonded to C, which is double bonded to O and single bonded to R.
A molecule R prime single bonded to C, which is double bonded to O and single bonded to R.
A molecule O single bonded to C, which is double bonded to R prime and single bonded to R.
A molecule R prime single bonded to C, which is single bonded to O and single bonded to R.
Answers
A structure diagram which completes the table of physical structures of the main functional groups is:
R'
|
C = O
|
R
What is a ketone?A ketone can be defined as a class of organic compound that typically comprises a carbonyl group in which an atom of carbon (C) is covalently bonded to an atom of oxygen (O).
In this context, we can infer and logically deduce that a structure diagram which completes the table of physical structures of the main functional groups is as follows:
R'
|
C = O
|
R
Read more on ketone here: https://brainly.com/question/23792559
#SPJ1

differentiate between true and metastable equilibrium
Answers
Answer:
True equilibriums are stable and they are always what it says it is in the scientific data and or experiments after the proof.
Metastable equilibriums are somewhat stable, but they tend to change their course at times. They will go up and down without ever really settling in one place very long. They vary.
Hopefully this helps! Feel free to mark as brainliest!
Explanation:
Identify the two gases jn the unknown mixtures.

Answers
Answer:
I believe Gases A and D
Explanation:
The lines in both gases match up with the lines in the unknown mixture.
Answer:
Fa (rt) and Nitrogen
Explanation:
How can you determine if a circuit is a series circuit or a parallel circuit?
Answers
Answer: In a parallel circuit, all components are connected across each other, forming exactly two sets of electrically common points.
Explanation: