What is the effect of the following on the volume of 1 mol of an ideal gas?
(b) The temperature changes from 305 K to 32°C, and the pressure changes from 2 atm to 101 kPa.
Answers
Boyle's law states that a gas's pressure and the volume it occupies are inversely proportional. Therefore, the correct answer is Volume increases by 100% under the conditions mentioned in the question.
An Ideal gas in which all collisions between atoms or molecules are perfectly elastic and in which there are no intermolecular attractive forces
PV = nRT
(P1 V1) / T1 = (P2 V2)/ T2
(2atm × V1)/ 305 = (0.996792×V2) / 305.15
0.006557×V1 = 0.003266×V2
V2 = 2V1
Therefore, the volume doubled and increased by 100%.
Learn more about Volume here:
brainly.com/question/5018408
#SPJ4
Related Questions
Help me please before 11:59 tmr

Answers
A fuel like natural gas is burned by a lot of hot water heaters. Because combustion is an exothermic reaction, the fuel's burning causes the water to heat up.
What is an exothermic reaction?Exothermic simply means "emitting heat." As an exothermic process develops, energy, frequently in the form of heat, is released.Energy is continuously released during an exothermic reaction, frequently in the form of heat.When new bonds form in the products of some chemical reactions, known as endothermic reactions, less energy is released than is required to break bonds in the reactants.Exothermic reactions characterize all combustion processes. A substance burns as it reacts with oxygen during combustion, releasing energy in the form of heat and light.Chemical processes known as exothermic reactions generate heat. when heat is transferred from the system to the environment. Therefore, the H of reaction is negative for exothermic processes.Exothermic reactions are those in which heat is released through chemical processes. Examples include a candle burning and a strong acid reacting with water.To learn more about exothermic reaction refer to:
https://brainly.com/question/2924714
#SPJ1
How do diffusion and osmosis differ?
Thank you
Answers
Answer:
Osmosis is the movement of solvent particles across a semipermeable membrane from a dilute solution into a concentrated solution. ... Diffusion: Diffusion is the movement of particles from an area of higher concentration to lower concentration. The overall effect is to equalize concentration throughout the medium.
Which of the following statements are true of the maintenance of blood pressure (BP)?
Answers
C. If blood pressure decreases, filtration in the kidneys decreases.
D. If more blood returns to the heart, the ventricles contract more forcefully to pump it out, and this raises blood pressure.
What is the maintenance of blood pressure?The maintenance of blood pressure is defined as the high use of sodium in your diet and the low use of potassium in your diet can cause hypertension. It is also important to use those foods which are free from fats. It is also important that we should use fruits, vegetables, and grains in excessive amounts for the maintenance of blood pressure and for a healthy diet.
High blood pressure 190/110 mm Hg can cause the main vital organs of the body like the brain, heart, and kidney too. There are three factors that contribute to blood pressure which are resistance, blood viscosity, and blood vessel diameter.
So we can conclude that blood pressure is the pressure of circulating blood against the walls of the blood vessels.
Learn more about Blood Pressure here: https://brainly.com/question/25149738
#SPJ1
Which of the following statements are true of the maintenance
of blood pressure (BP)? (Read carefully and select all of the correct statements.)
A. Norepinephrine stimulates vasoconstriction in skin, viscera, and skeletal muscles, all of which lower BP.
B. Aldosterone stimulates the reabsorption of Na+ ions by the kidneys, which lowers BP.
C. If BP decreases, filtration in the kidneys decreases.
D. If more blood returns to the heart, the ventricles contract more forcefully to pump it out, and this raises BP.
E. If BP decreases, the kidneys secrete the enzyme renin, which stimulates the secretion of epinephrine to prevent a further decrease.
F. The hormone ANP increases the excretion of K+ ions by the kidneys, which helps conserve water and raise BP.
G. The hormone ADH helps the kidneys conserve water and helps prevent a decrease in BP.
H. The elasticity of the large arteries helps decrease diastolic BP.
A chemist wants to extract copper metal from copper chloride solution. The chemist places 0. 25 grams of aluminum foil in a solution of 0. 40 grams of copper (II) chloride. A single replacement reaction takes place. What are the likely observations when the reaction stops?
Unbalanced equation: CuCl2 + Al - AlCl3 + Cu
Answers
The chemist should observe the formation of solid copper metal, a change in color of the solution, and the disappearance of the aluminium foil through this unbalanced equation.
The balanced equation for the reaction between copper (II) chloride and aluminum is:
3CuCl2 + 2Al → 2AlCl3 + 3Cu
In this reaction, aluminum replaces copper in the copper chloride solution to form aluminium chloride and copper metal.
The likely observations when the reaction stops are:
1. The solution may change color, indicating that a chemical reaction has occurred. Copper (II) chloride is blue, while aluminum chloride is colorless.
2. Solid copper metal may form and settle at the bottom of the container, indicating that a precipitation reaction has occurred.
3. The aluminum foil may appear to have dissolved or disintegrated, as it has been consumed in the reaction.
4. The reaction mixture may become warmer due to the exothermic nature of the reaction.
To learn more about unbalanced equation Click here:
brainly.com/question/11322377
#SPJ4
true/false. Given the equation representing a reaction at equilibrium: N2(g) 3H2(g) ( 2NH3(g) Explain, in terms of collision theory, why the rate of the forward reaction decreases when the concentration of N2(g) is decreased.
Answers
Less actual collisions. Because there is fewer N₂ molecule to collision with H₂ molecules, the forward reaction's pace slows down. Because crashes happen less frequently, the rate decreases.
What does the term "reaction" in chemistry mean?In a chemical reaction, one or more chemicals, usually known as reactants, change into same or more other compounds, frequently referred to as products.
What are reactions and how do they differ?Pairing, decomposition, fixed, double-replacement, and conflagration are the five fundamental kinds of chemical processes. You may classify a reaction into one of these groups by looking at the reactants and products. Several categories will apply to some reactions.
To know more about Reaction visit:
brainly.com/question/25769000
#SPJ4
The complete question is-
Given the equation representing a reaction at equilibrium:
N₂(g) + 3H₂(g) <==>2NH₃(g)
Explain, in terms of collision theory, why the rate of the forward reaction decreases when the concentration of N₂(g) is decreased.
What is the molarity of a solution that has 90 mol of solute dissolved in 6 liters of solvent? ___ M
Answers
Answer:
15M
Explanation:
To find molarity, you use the equation :
Molarity = \(\frac{moles of solute}{liters of a solution}\)
So to find the molarity, you would do 90mols of the solute divided by 6 liters of the solvent which is 15M.
The solubility of solid substances generally ___________ as temperature increases. The solubility of gaseous substances generally ___________ as temperature increases. Group of answer choices
Answers
The solubility of solid substances generally increases, while the solubility of gaseous substances decreases with temperature.
How does temperature affect solubility?The solubility of solid substances generally increases as temperature increases. This is because increasing the temperature provides more energy to the particles of the solid, causing them to move faster and collide more frequently with the solvent particles. These increased collisions facilitate the dissolution process and enhance the solubility of the solid in the solvent.
This phenomenon is commonly observed in many solid solutes such as sugar, salt, and various salts in water.
On the other hand, the solubility of gaseous substances generally decreases as temperature increases. This can be explained by the fact that gases are more soluble at lower temperatures because the kinetic energy of the gas particles decreases, leading to weaker molecular interactions.
As the temperature rises, gas particles gain more energy, causing them to move more rapidly and exert a greater pressure on the solvent. This higher pressure reduces the solubility of the gas, resulting in its release from the solution as bubbles or gas phases.
It is important to note that while these general trends hold true for many substances, there can be exceptions depending on the specific chemical properties and interactions involved in the solvation process.
Learn more about Temperature and solubility
brainly.com/question/12976543
#SPJ11
does a polymerization of ethylene units will result in the polymer polyethylene?
Answers
Yes, the polymerization of ethylene units will result in the polymer polyethylene.
Polyethylene is a common thermoplastic polymer made from the polymerization of ethylene monomer units. Ethylene (C₂H₄) has a double bond between the carbon atoms, which can be broken under certain conditions to form new single bonds. When many ethylene molecules undergo this reaction, they can polymerize into long chains of polyethylene molecules.
The resulting polyethylene polymer has a high molecular weight and is a widely used plastic material due to its properties such as strength, flexibility, and chemical resistance.
To learn more about polymer polyethylene refer to:
brainly.com/question/15265692
#SPJ4
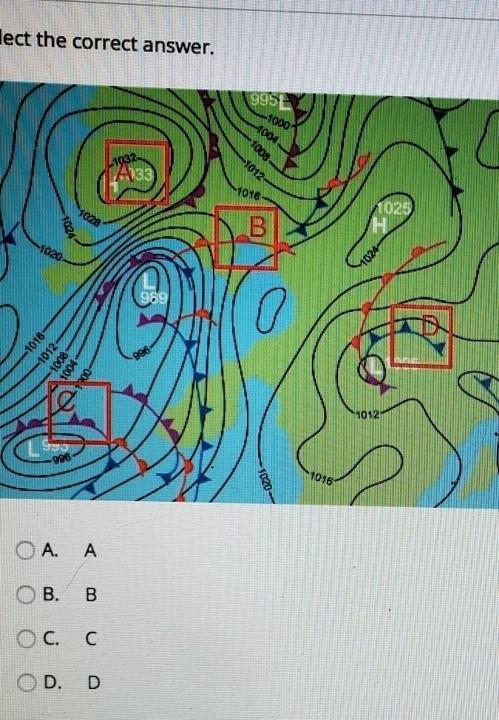
Select the correct answer.
Map representing Low and High wind pressure areas. Also, has A, B, C, and D in a red color box marked at places.On the map, which symbol represents a cold front? Choose the correct letter.
A.
A
B.
B
C.
C
D.
D
Answers
The correct answer is D. In any weather/climate map, a cold front is depicted by a blue hue and blue triangles pointing the direction it will be travelling toward/impacting.
What is a cold front?A cold front is the leading edge of a cooler mass of air at ground level that replaces a warmer mass of air and rests within a noticeable low-pressure trough.
It frequently originates behind an extratropical cyclone, along the leading edge of the storm's cold air advection pattern, also known as the cyclone's dry "conveyor belt" flow.
When a somewhat wet, warm air mass flows up and over a cold air mass, a Warm Front occurs. When warm air rises, it frequently condenses into a large region of clouds. The warm air at the surface advances steadily behind the warm front, replacing the cold air at the surface.
Learn more about cold front:
https://brainly.com/question/29770786
#SPJ1
Full question
See attached image.
A 28.94 L quantity of neon gas is at 20.0°C when some punk bunny turns thdown to 10.0°C. What is the new volume of the neon gas?Record the number value of your answer to two decimal places.

Answers
27.95L
Explanations:According to Charle's law, the volume of a given mass of a gas is directly proportional to absolute temperature provided that the pressure is constant. Mathematically;
\(\begin{gathered} V\alpha T \\ V=kT \\ k=\frac{V_1}{T_1}=\frac{V_2}{T_2} \end{gathered}\)where
V1 and V2 are the initial and final volume respectively
T1 and T2 are the initial and final temperature respectively (in Kelvin)
Given the following parameters
\(\begin{gathered} V_1=28.94L \\ T_1=20.0^0C+273=293K \\ T_2=10.0^0C+273=283K \end{gathered}\)Required
Final volume V₂
Substitute the given parameters into the formula to have:
\(\begin{gathered} V_2=\frac{V_1T_2}{T_1} \\ V_2=\frac{28.94\times283}{293} \\ V_2=\frac{8190.02}{293} \\ V_2=27.95L \end{gathered}\)Hence the new volume of the neon gas is 27.95L
How do you find the molar mass of CaSO4 2H2O?
Answers
To find the molar mass of CaSO₄ 2H₂O, you need to add the atomic masses of all the elements present in the compound. The molar mass of CaSO₄ 2H₂O is 146.24 g/mol.
The molar mass of a substance is the mass of one mole of the substance and is expressed in units of grams per mole (g/mol). To calculate the molar mass of CaSO₄ 2H₂O, you need to know the atomic masses of calcium (Ca), sulfur (S), oxygen (O), and hydrogen (H).
Calcium has an atomic mass of 40.08 g/mol, sulfur has an atomic mass of 32.06 g/mol, oxygen has an atomic mass of 16.00 g/mol, and hydrogen has an atomic mass of 1.01 g/mol. To find the molar mass of CaSO₄ 2H₂O, you need to multiply the number of each element in the compound by its atomic mass and then add up all of the masses.
CaSO₄ 2H₂O, which means that there is one calcium atom, one sulfur atom, four oxygen atoms, and ten hydrogen atoms in the compound. Multiplying the number of each element by its atomic mass and adding up all the masses gives you the molar mass of CaSO₄ 2H₂O:
(1 x 40.08 g/mol) + (1 x 32.06 g/mol) + (4 x 16.00 g/mol) + (10 x 1.01 g/mol) = 40.08 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol = 146.24 g/mol
To know more about molar mass here:
https://brainly.com/question/12127540#
#SPJ11
In a car piston shown above the pressure of the compressed gas (red) is 5.00 atm if the area of the piston is 0.0760 m2 what is the forces exerted by the gas on the piston in newtons (N)
Answers
The force exerted by the gas on the piston in newtons (N) is 38503.5N.
How to calculate force from pressure?Pressure is the amount of force that is applied over a given area divided by the size of this area.
This means that the pressure can be calculated as follows:
P = F/A
Where;
P = pressure (Pa or N/m²)F = force (N)A = area (m²)According to this question, in a car piston, the pressure of the compressed gas (red) is 5.00 atm. If the area of the piston is 0.0760 m², the force exerted can be calculated as follows:
506625N/m² = F/0.0760m²
F = 38503.5N
Therefore, the force in Newtons is 38503.5N.
Learn more about pressure at: https://brainly.com/question/28012687
#SPJ1
what is the answer ?????????

Answers
What a camera does is capture and record the image of objects
Operations of a cameraA camera is a device that is used to capture or record the image of objects.
There are two types of camera:
Still camerasMotion or video camerasStill cameras capture and record images while motion cameras capture and record moving images of objects or people.
Cameras generally work using a system of lenses that capture rays of light from objects and redirect them to a single point - a sensor of film - where images of the objects are created.
More on cameras can be found here: https://brainly.com/question/9405339?referrer=searchResults
SOMEBODY PLEASE HELP MEE !!!

Answers
How can you use probability to predict the outcome of a codominant cross?
Answers
Featured snippet from the web
Calculate: Punnett squares can be used to predict probable outcomes of genetic crosses. To calculate probability, divide the number of one kind of possible outcome by the total number of all possible outcomes. For example, if you toss a coin, the chance it will land on heads is equal to 1 ÷ 2.
An indicator is used in a titration toshow when _It does this bychanging color.A there has been a change in temperatureB. to add more waterC. an equal number of moles of acid and base are present

Answers
ANSWER
EXPLANATION
Firstly, we need to define the word titration.
Titration is defined as a technique that is used to determine the known concentration of an unknown solution.
This normally occurs between an acid and a base
During titration, an indicator changes color when equilibrium has been attained between the two solutions. The solutions are normally acid and base. At equilibrium, the number of moles of acid is equal to the number of base.
Therefore, the correct answer is option C
1. A cell can be seen by looking through a microscope. Seeing which of these organelles
would let you know that you are looking at a plant cell?
A. Mitochondria
B. Chloroplast
C. Cell membrane
D. Nucleus
Answers
How many molecules are in 7V205?
Answers
Answer:
Calculate the number of moles you have by taking the Mass / molar mass. if you have 1000 grams ; then 1,000 g / 151.001 g/mol = X g moles. Then multiply by Avogadros # = 6.022140857 × 10^23 molecules per g mole. The result is the # of molecules of MnSO4
Explanation: Hope this helps
given the chemical reaction co2 + h2o = hco3- + h+, an increase in co2 leads to ______.
Answers
The chemical reaction CO2 + H2O = HCO3- + H+ is an important reaction in the regulation of the pH of blood and other bodily fluids.
This reaction occurs in the red blood cells and involves the conversion of carbon dioxide (CO2) and water (H2O) into bicarbonate ion (HCO3-) and hydrogen ion (H+).
An increase in CO2 will lead to an increase in the concentration of H+ ions and HCO3- ions in the blood.
This is because CO2 is an acidic gas, and when it dissolves in water, it forms carbonic acid (H2CO3). Carbonic acid then dissociates into H+ ions and HCO3- ions, increasing the concentration of both ions in the blood.
This increase in H+ ions will cause a decrease in the pH of the blood, making it more acidic.
This increase in acidity can have negative effects on the body, such as interfering with enzyme activity and altering protein structure.
The body has mechanisms in place to regulate the pH of the blood and other bodily fluids, such as the respiratory and renal systems, which can help to compensate for changes in CO2 levels and maintain a stable pH.
To know more about CO2 + H2O = HCO3- + H+ refer here
brainly.com/question/10958730#
#SPJ11
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
please help me...(╥﹏╥)

Answers
I don't know who is our time together in the topic of your name and hygiene and tropical storm is not a problem with the topic of your name and address of the topic of your name and address of the topic of your name and address of the topic of your name and address of the topic of your name and address of the topic
PLEASE SHOW WORK PLEASE !!!! need help
Question 7 Calculate the pH of 0.81 M Mg(OH)₂. Show your work to earn points. Use the editor to format your answer Question 8 Calculate the pH of 0.27 M solution of the pyridine (CsHsN; K=1.7 x 10%)
Answers
7. the pH of 0.81 M Mg(OH)₂ solution is 9.19.
8. the pH of 0.27 M pyridine solution is 9.11.
Mg(OH)₂ is a base which dissociates to produce two OH⁻ ions.
Mg(OH)₂ → Mg²⁺ + 2 OH⁻
Let the concentration of OH⁻ ions produced be x.
Therefore, the concentration of Mg²⁺ is 0.81-x
Mg(OH)₂ → Mg²⁺ + 2 OH⁻
Initial concentration (M) 0 0
Change (M) -x +2x
Equilibrium Concentration 0.81-x x x
Using Kb for Mg(OH)₂,Kb = Kw/Ka
Kw = 1.0 × 10⁻¹⁴ at 25 °C.
For Mg(OH)₂,Kb = [Mg²⁺][OH⁻]²/Kw= (x)²/0.81 - x
Kb = 4.5 × 10⁻¹² = x²/0.81 - x
On solving the equation,x = 7.7 × 10⁻⁶M
Therefore, the concentration of OH⁻ ions = 2 × 7.7 × 10⁻⁶ = 1.54 × 10⁻⁵ M
To calculate the pH of the solution, use the formula:
pOH = - log [OH⁻]= - log 1.54 × 10⁻⁵pOH = 4.81pH = 14 - 4.81 = 9.19
Thus, the pH of 0.81 M Mg(OH)₂ solution is 9.19.
Let the concentration of OH⁻ ions produced be x.
Therefore, the concentration of C₅H₅NH⁺ is 0.27 - x.
C₅H₅N + H₂O ⇌ C₅H₅NH⁺ + OH⁻
Initial concentration (M) 0.27 0
Change (M) -x +x
Equilibrium Concentration 0.27-x x
Using Kb for C₅H₅N,Kb = Kw/Ka
Kw = 1.0 × 10⁻¹⁴ at 25 °C.
For C₅H₅N,
Kb = [C₅H₅NH⁺][OH⁻]/[C₅H₅N]= (x) (x)/(0.27-x)Kb = 1.7 × 10⁻⁹
= x²/(0.27-x)
On solving the equation,
x = 1.3 × 10⁻⁵ M
Therefore, the concentration of OH⁻ ions = 1.3 × 10⁻⁵ M
To calculate the pH of the solution, use the formula:
pOH = - log [OH⁻]= - log 1.3 × 10⁻⁵pOH
= 4.89pH = 14 - 4.89 = 9.11
Thus, the pH of 0.27 M pyridine solution is 9.11.
learn more about pH here
https://brainly.com/question/12609985
#SPJ11
which explains how the nervous system is typically involved in keeping the body in Homeostasis?

Answers
Answer:
c because this is the one hundred all the time
Explanation:

Next to a shallow cylindrical lake with a radius of 4km and an average water height of 5m, a type A exhaust basin has been installed, which recorded a total water loss of 4.5cm during a summer month. It is requested to calculate the evaporation of the lake and the volume of the lake water in cubic meters for the specific time period if the coefficient of the evaporation basin is equal to 0.7
Answers
In a shallow cylindrical lake with a radius of 4 km and an average water height of 5 m, a type A exhaust basin recorded a total water loss of 4.5 cm during a summer month.
The task is to calculate the evaporation of the lake and the volume of lake water in cubic meters for that specific time period, assuming an evaporation coefficient of 0.7. To calculate the evaporation of the lake, we first convert the recorded water loss from centimeters to meters. The total water loss is 4.5 cm, which is equal to 0.045 meters.
The evaporation from the lake can be determined by multiplying the water loss by the evaporation coefficient. In this case, the evaporation coefficient is given as 0.7. So, the evaporation from the lake is calculated as:
Evaporation = Water loss * Evaporation coefficient
Evaporation = 0.045 m * 0.7 = 0.0315 m
Therefore, the evaporation of the lake during the specified time period is 0.0315 cubic meters.To calculate the volume of lake water, we need to consider the shape of the lake, which is a shallow cylinder. The formula for the volume of a cylinder is:
Volume = π * radius^2 * height
Given that the radius of the lake is 4 km (4000 m) and the average water height is 5 m, we can calculate the volume of the lake as:
Volume = π * (4000 m)^2 * 5 m = 251,327,412 m^3
Therefore, the volume of lake water for the specific time period is approximately 251,327,412 cubic meters.
Learn more about evaporation here:- brainly.com/question/28319650
#SPJ11
Rowena and helga are both performing an experiment with nickel metal. Rowena has a 5 gram sample and determines the density to be 8.9g/cm3. If helga has a nickel sample that is twice as large and has a mass of 10 grams what would be the density of helgas sample?
Answers
Answer:
The density of helgas sample is 17.8 g/cm³.
Explanation:
Given that,
Mass of sample of Rowena = 5 gram
Density = 8.9 g/cm³
Mass of sample of helga = 10 gram
We need to calculate the volume of sample
Using formula of volume
\(V=\dfrac{m}{\rho}\)
Where, m = mass
\(\rho\) = density
Put the value into the formula
\(V=\dfrac{5}{8.9}\)
\(V=0.56\ cm^3\)
We need to calculate the density of helgas sample
Using formula of density
\(\rho=\dfrac{m}{V}\)
Where, m = mass
V = volume
Put the value into the formula
\(\rho=\dfrac{10}{0.56}\)
\(\rho=17.8\ g/cm^3\)
Hence, The density of helgas sample is 17.8 g/cm³.
what would the molarity of the dichromate in the volumetric flash before it reactswith the fe (ii) in thesample?
Answers
To determine the molarity of the dichromate in the volumetric flask before it reacts with the Fe(II) in the sample, please follow these steps.
1. Identify the initial concentration and volume of the dichromate solution. This information is usually given in the problem or can be found through a series of calculations.
2. Calculate the moles of dichromate ions using the initial concentration and volume. To do this, use the formula: moles = concentration x volume.
3. Find the volume of the volumetric flask. This information is typically given in the problem or can be measured.
4. Determine the molarity of the dichromate in the volumetric flask. To do this, use the formula: molarity = moles / volume of the flask.
By following these steps, you can determine the molarity of the dichromate in the volumetric flask before it reacts with the Fe(II) in the sample.
To know kore about molarity, refer
https://brainly.com/question/30404105
#SPJ11
PLEASE HELP!!!! Explain the process that causes dew to form on blades of grass
Answers
Answer:
Explanation:
Dew forms when the object, such as the glass, cools down to the dew point temperature. Water molecules in the air continually bombard surfaces, like blades of grass If the object gets cold enough, and the air contains enough moisture, condensation exceeds evaporation, and the film grows into dew drops.
3. SEP Identify Patterns How did you classify reaction 4? Based on periodic
patterns (the available electrons to form bonds), provide an explanation for the
sodium chloride product formed.
Answers
Reaction 4 is a type of chemical reaction called a precipitation reaction, which involves the formation of a solid (precipitate) when two solutions are mixed. In this reaction, sodium hydroxide (NaOH) and hydrochloric acid (HCl) are mixed, resulting in the formation of sodium chloride (NaCl) and water (H2O).
The formation of sodium chloride can be explained based on periodic patterns of available electrons to form bonds. Sodium (Na) has one valence electron and chlorine (Cl) has seven valence electrons. Sodium tends to lose one electron to achieve a stable octet configuration, while chlorine tends to gain one electron to achieve a stable octet configuration. Therefore, in the reaction between NaOH and HCl, the sodium ion (Na+) and chloride ion (Cl-) combine to form NaCl, which is held together by ionic bonds due to the electrostatic attraction between the positively charged Na+ and negatively charged Cl- ions. This reaction follows the periodic pattern of the reactivity of elements based on their electron configuration.
To know more about precipitation reaction click here:
https://brainly.com/question/29762381
#SPJ11
Typically, nitrogen atoms are composed of electrons, protons, and neutrons. an isotope of nitrogen could?
Answers
Typically, nitrogen atoms are composed of electrons, protons, and neutrons. an isotope of nitrogen could have more number of neutrons than usual nitrogen atom.
What is proton ?
Protons include the H+ ion or the hydrogen atom's nucleus. Regardless of the isotope, each hydrogen atom has one proton, each helium atom has two, each lithium atom has three, and so on.
What is electron ?
It is possible for an electron to be free or linked to an atom. An electron is a negatively charged subatomic particle (not bound). There are three main types of particles in an atom: protons, neutrons, and an electron that is bonded to an atom. The nucleus of an atom is composed of protons, neutrons, and electrons.
Therefore, nitrogen atoms are composed of electrons, protons, and neutrons. an isotope of nitrogen could have more number of neutrons than usual nitrogen atom.
Learn more about proton from the given link.
https://brainly.com/question/17351413
#SPJ4
What is a result of the unequal electron sharing in a water molecule? (5 points)
Water molecules have a nonpolar bond.
Water molecules have a weakly positive oxygen end.
Water molecules have a weakly positive hydrogen end.
Water molecules have two oxygen and two hydrogen atoms.
Answers
You may be wondering why I have to right so much it’s because their a word count requirement.
Answer:
Water molecules have a weakly positive hydrogen end.
Explanation:
Water is a polar molecule. The pairs of electrons shared by the hydrogen atoms in a water molecule are actually more attracted to the oxygen atom with its eight protons than to the hydrogen atoms with their one each. The hydrogen end of the molecule is weakly positive and the oxygen end is weakly negative.
I hope it helps.