What is the name of the compound present in all aqueous systems involving brønsted-lowry acids or bases?.
Answers
The name of the compound present in all aqueous systems involving Brønsted-Lowry acids or bases is the hydronium ion.
Brønsted-Lowry acid-base theory defines an acid as a proton (H⁺) donor and a base as a proton acceptor. The acid dissociates in water and passes a proton to a base, forming its conjugate base.
In aqueous solutions, hydrogen ions (H⁺) do not exist as isolated entities but are bonded to a molecule of water, which gives the hydronium ion (H₃O⁺). When an acid is added to water, it transfers its proton to a water molecule to form the hydronium ion, which is present in all aqueous systems involving Brønsted-Lowry acids or bases. Similarly, when a base is added to water, it accepts a proton from a water molecule to form hydroxide ions (OH⁻).
The compound present in all aqueous systems involving Brønsted-Lowry acids or bases is the hydronium ion (H₃O⁺).
To know more about Brønsted-Lowry acids, visit:
https://brainly.com/question/32276007
#SPJ11
Related Questions
What is the mass of 1.55 x 10^24 molecules of chlorine?
Answers
Explanation:
Use the molecular formula to find the molar mass; to obtain the number of moles, divide the mass of compound by the molar mass of the compound expressed in grams.
Answers for this assignment

Answers
The following chemical compounds are;
V₂O₅ Vanadium (V) oxide,
SF₆ Sulfur hexafluoride
HClO₂ Chlorous acid
(NH₄)₂SO₄·6H₂O Ammonium sulfate hexahydrate
What should you know about the chemical compound called Chlorous acid?Chlorous acid is a weak and unstable acid. This acid exists mainly in aqueous solutions. They are used in disinfectants, and bleaching products. They are good sanitizers and helps to reduce bacteria.
Apart from being in products we commonly use in our house, Chlorous acid is also used in the paper and textile industries for bleaching purposes.
Find more exercises on Chlorous acid;
https://brainly.com/question/15084224
#SPJ1
What type of reaction occurs between acetic acid and sodium bicarbonate in the Titration Lab?
Answers
Answer:
Explanation:
Titration is
when in a lab someone uses
a small pipe called a
pipette
and
drop by drop
they add chemical B (titrant) to chemical A
until something happens
its done drop by drop to find out what is the exact amount needed to cause a reaction
sciencedirectcom
Answer:
Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and sodium acetate.
Explanation:
Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and sodium acetate. The solid baking soda was placed in liquid vinegar producing carbon dioxide gas, which is evident because of the formation of bubbles in the foaming mixture.
Gallium changes it’s state of matter from solid to liquid in someone’s hand. Think about other substances that you are familiar with that change state. Ice melts in the Sun, and soup steams when it boils. Are you starting to get some ideas on why materials change state? Use gallium as an example to make a claim about what causes a substance d to change states
CLAIM
Gallium changes state because…
Answers
A matter can change from one state to another by absorbing or losing energy. Some of the example of such changes are melting, boiling, freezing, etc. Here 'Ga' changes into liquid state at high temperature.
What are states of matter?The three states of matter represents the three distinct physical forms in which matter can take in most environments. The common states of matter are solid, liquid and gas. A change of state is a physical change in the matter.
When the temperature or pressure of a system changes, then there occurs a change of state. The intermolecular interaction between the molecules increases with the increase in temperature and pressure. Similarly when the temperature decreases, molecules form a rigid structure.
Thus a change of state occurs on changing some parameters.
To know more about states of matter, visit;
https://brainly.com/question/9402776
#SPJ1
ANSWER PLS ASAP Fireworks are an example of a
amount of energy being

Answers
Answer:
large, released
Explanation:
As we know, fireworks contain l o t s of energy, even before the burst of colors release. So i think choice 3 is the answer.
i hope this helps :)
Convert 550 Hm to mm
Answers
Answer:
55,000,000
Explanation:
550 hectometers [hm] = 55,000,000 millimeters [mm]
PLSSSSS HELP YALL!! THIS IS WORTH 60 PTS
PLSSS HELP
1. The Punnett Square shows the cross between two parents who are carriers for sickle cell anemia, a recessive genetic disorder that affects the red blood cells in humans.
The probability that these parents will have an offspring affected with sickle cell anemia is ______.
A. 50
B. 75
C. 100
D. 125
2. Using the same Punnett Square, what is the probability that the offspring will be carriers like the parents?
A. 100
B. 25
C. 75
D. 50

Answers
Answer:
Question 1 - 25%
Question 2 - 50%
Explanation:
Question 1:
Because sickle cell anemia is a recessive disorder, any genotype with two recessive (ss) alleles will represent the phenotype. Because the Punnett square shows only one ss genotype out of four, the probability that the parents will have an offspring affected with sickle cell anemia is 25%
Question 2:
To be a carrier of a genetic trait, one must both have the dominant and recessive alleles (Ss). Because there are two Ss genotype out of four, the probability that the parents will have offspring affected with sickle cell anemia is 50%
Answer:
Question 1 - 25%
Question 2 - 50%
Explanation:
If 10 moles of P4S3 was used, how many grams of P4O6 was produced? Leave up to 3 decimal places when possible.
Answers
If 10 moles of P4S3 were used, the mass of P4O6 produced would be 2838.8 grams.
To determine the number of grams of P4O6 produced from 10 moles of P4S3, we need to use the balanced chemical equation and the molar masses of the compounds involved.The balanced equation for the reaction between P4S3 and oxygen to produce P4O6 is:
P4S3 + 8 O2 → P4O6 + 6 SO2
From the balanced equation, we can see that the molar ratio between P4S3 and P4O6 is 1:1. This means that for every 1 mole of P4S3 consumed, 1 mole of P4O6 is produced.The molar mass of P4S3 is 220.25 g/mol, and the molar mass of P4O6 is 283.88 g/mol.
To calculate the mass of P4O6 produced, we can use the following equation:
Mass of P4O6 = Moles of P4O6 × Molar mass of P4O6
Since the molar ratio between P4S3 and P4O6 is 1:1, the number of moles of P4O6 produced is also 10 moles.
Mass of P4O6 = 10 moles × 283.88 g/mol = 2838.8 grams
for such more questions on mass
https://brainly.com/question/24191825
#SPJ8
A substance dissociates into K+ and Cl– in solution. The substance is a(n) ________.
a. acid
b. base
c. salt
d. buffer
Answers
The substance that dissociates into K⁺ and Cl⁻ in solution is a salt. Therefore, the correct answer is option c. salt.
In this case, the substance is potassium chloride (KCl), which is an ionic compound formed by the combination of a metal cation (K⁺) and a non-metal anion (Cl⁻).
KCl, or potassium chloride, is a chemical compound that is commonly known as a salt. It consists of potassium (K+) ions and chloride (Cl-) ions. The compound is formed through the ionic bonding between the positively charged potassium ion and the negatively charged chloride ion.
KCl is an odorless and colorless crystalline solid that has a salty taste, similar to common table salt (NaCl). It is highly soluble in water and forms a clear solution.
Learn more about Salt from the link given below.
https://brainly.com/question/31814919
#SPJ4
Up late, a tired engineer calculated the molar mass of nitroglycerin (C3H5N3O9) to be 193.7 g/mol, but this incorrect. What is the percent error of this incorrect molar mass
Answers
So, the formula mass of nitroglycerin is\(3(12.011)+5(1.00794)+3(14.0007)+9(15.9994)=227.0694 \text{ g/mol}}\)
Therefore, the percent error is
\(\frac{193.7-227.0694}{227.0694} \times 100=\boxed{-14.70 \% \text{ (to 4 sf)}}\)
two example of solid -solid solution
Answers
Answer:
Steel — in steel, there is a solid-solid solution of iron and carbonBrass — in brass, there is a solid-solid solution of zinc and copper.24. In our experiment, we filled 4 tubes with water and bromothymol blue.
Tube 1 did not contain an organisms, tube 2 contained a snail, tube 3
contained a elodea and tube 4 contained both a snail and elodea. What
tubes could photosynthesis occur in?
O Tubes 3 and 4
O Tubes 2 and 4
O Tubes 2, 3, and 4
Answers
Answer:
C. Tubes 2, 3, and 4
Explanation:
Tube one doesn't contain an organism so it can't be that one.
Both a snail and an elodea are considered an organism, cellular respiration occurs in almost any organism's cells.
good luck, i hope this helps :)
In the common naming convention for carboxylic acids, what is the correct greek letter used for the carbon adjacent to the carboxyl group?.
Answers
The correct Greek letter used for the carbon adjacent to carboxyl group is alpha (α).
What is carboxylic acid?
Any of a group of organic compounds known as carboxylic acids in which a carbon (C) atom forms a double bond with an oxygen (O) atom and a single bond with a hydroxyl group (OH). The carbon atom is connected to a hydrogen (H) atom or another univalent combining group by a fourth bond. The carbonyl group (C=O) and hydroxyl group are what give the carboxyl (COOH) group its name. The acidity of the carboxylic acids is one‘s primary chemical characteristic. They are typically weaker than the well-known mineral acids but generally more acidic than other organic compounds with hydroxyl groups (e.g., hydrochloric acid, HCl, sulfuric acid, H2SO4, etc.).
Greek letter used for the carbon adjacent to the carboxyl group is Alpha.
To learn more about Carboxylic Acid from the given link.
https://brainly.com/question/26855500
#SPJ4
What is the complete reaction
Answers
Answer:
A reaction is "completed" when it has reached equilibrium — that is, when concentrations of the reactants and products are no longer changing. If the equilibrium constant is quite large, then the answer reduces to a simpler form: the reaction is completed when the concentration of a reactant falls to zero.
Explanation:
I hope it's help
Mark as brainliest answer
thanks me later
don't forget to follow me
I'm beginner plss support me
stay safe and healthy
What are the major disadvantages of using ozone instead of chlorine to disinfect water? O Ozonation is more expensive than chlorination and ozone leaves an odor in the water O Ozonation causes trihalomethane formation and leaves an odor in the treated water O Ozone decomposes quickly and does not provide long-term protection against possible contamination as the water is piped through a municipal distribution system O Ozonation causes trihalomethane formation and is more expensive than chlorination
Answers
The major disadvantage is - Ozone decomposes quickly and does not provide long-term protection against possible contamination as the water is piped through a municipal distribution system.
The gas ozone (O3) is unstable. As a result, it breaks down quickly, oxidizing any organic impurities present in the water including bacteria, viruses, and germs, but because it is a gas, it leaves the water after the oxidation is complete. Therefore, when the ozone started to disintegrate at first, the water may have various additional contaminants that mix with it as it travels through the municipal distribution system. Water carries through the new pollutants from the pipes. Ozone therefore has no long-term effect.
Chlorination produces trihalomethane, not ozonation, hence the expansiveness of ozone is dependent on the impurity of the water. Trihalomethane is produced by chlorination, not ozonation. Although ozone as a gas has a faint odor, when it is combined with water, it oxidizes the pollutant and breaks down, leaving no ozone in the water. Therefore, water does not smell like ozone.
Learn more about ozonation:
brainly.com/question/27911475
#SPJ4
What are 5 characteristics of ionic compounds?.
Answers
Characteristics of ionic compounds are
1. They develop crystals.
2. Both their melting and boiling points are very high.
3. They are fragile and hard.
4. Compared to molecular molecules, they have higher fusion and vapourization enthalpies. When dissolved in water, they act as an electrical conductor.
5. Ionic solids are excellent insulators because they do not conduct electricity.
What are ionic compounds ?An ionic compound in chemistry is a chemical complex made up of ions kept together by electrostatic forces known as ionic bonding. Although the molecule is mostly neutral, it contains positively charged ions known as cations and negatively charged ions known as anions.
The entire valence electron transfer between atoms is known as ionic bonding. Two ions with opposing charges are produced as a result of this kind of chemical connection. Ionic bonding result in a metal losing electrons to become a positively charged cation and a nonmetal accepting those electrons to become a negatively charged anion.Learn more about Ionic compounds here:
https://brainly.com/question/2687188
#SPJ4
Question 23 (1 point)
How many electrons are there in the third energy level of sodium, Na (atomic number 11)?
none
b
one
c
two
d
three
ine
Answers
Answer:
the answer is b, which is one
Is fresh squeezed lemonade a homogeneous or heterogeneous mixture
Answers
Answer: This makes the lemonade a homogenous mixture
Explanation:
If the freshly squeezed lemonade is made by squeezing lemons to extract the sour juice and mixing this with water and sugar all the substances in the mixture finally created are in the same phase.
What does a coordination number tell you?
Answers
Answer:
45
Explanation:
kasi madali lng sya buhatun dpat paning kamotan nimo na sili kay magsalig ka sa brainly dahh
The reaction between a strong acid and a weak base produces a salt, but water is not usually formed because:
the reaction is too hot and water evaporates
there is no hydrogen present to form water
the acid is not strong enough to form water
weak bases tend not to be hydroxides
Answers
Answer:
3
Explanation:
Why does a planet stay in orbit?
Answers
Answer:
Gravity
Explanation:
A planet is gravitationally pulled around the sun.
Answer:
beause of gravitys pull and because the atmosphere of the beyond lol also bcs this i hope this helps your welcome boo >3
Explanation:
Newton realized that the reason the planets orbit the Sun is related to why objects fall to Earth when we drop them. The Sun's gravity pulls on the planets, just as Earth's gravity pulls down anything that is not held up by some other force and keeps you and me on the ground.
Gasohol is a mixture of gasoline and ethanol (grain alcohol), C2H5OH. Calculate the maximum work that could be obtained at 25 °C and 1 atm by burning 1. 003 mol of C2H5OH. C2H5OH(1) + 302(g) 2C02(g) + 3H20(g) Maximum work = kJ Use correct number of significant digits;
Answers
Gasohol is a blend of gasoline and ethanol. To determine the maximum work that can be obtained by burning 1.003 mol of C2H5OH at 25°C and 1 atm, the Gibbs free energy equation can be utilized. What is Gibbs free energy equation? Gibbs free energy equation is a thermodynamic equation that quantifies the maximum quantity of work that may be obtained during a chemical reaction. T
he equation is as follows: ΔG = ΔH - TΔSThe values of ΔH and ΔS are calculated from thermodynamic tables or by calculating the enthalpy and entropy of the products and reactants, and the temperature, T, is usually specified in Kelvin. The change in Gibbs free energy, ΔG, is the maximum amount of energy that can be obtained from the reaction in the form of useful work if the reaction takes place at constant pressure and temperature. The reaction will proceed spontaneously if ΔG is negative. And if ΔG is positive, the reaction will not take place spontaneously. The solution to this problem is shown below:
First, let's figure out how much heat is produced when one mole of C2H5OH is burnt.ΔHrxn = [2(moles of CO2)(-393.5 kJ/mol) + 3(moles of H2O)(-285.8 kJ/mol)] - [(moles of C2H5OH)(-277.7 kJ/mol)]ΔHrxn = [2(2.006 mol)(-393.5 kJ/mol) + 3(3.009 mol)(-285.8 kJ/mol)] - [1.003 mol(-277.7 kJ/mol)]ΔHrxn = -2043.5 kJ/mol. Now, we'll figure out the entropy change for the reaction.ΔSrxn = [2(moles of CO2)(213.8 J/mol-K) + 3(moles of H2O)(69.9 J/mol-K)] - [(moles of C2H5OH)(160.7 J/mol-K)]ΔSrxn = [2(2.006 mol)(213.8 J/mol-K) + 3(3.009 mol)(69.9 J/mol-K)] - [1.003 mol(160.7 J/mol-K)]ΔSrxn = -104.3 J/mol-KThe temperature in Kelvin is 25°C.273 + 25 = 298 KΔG = ΔH - TΔSΔG = -2043.5 kJ/mol - (298 K)(-104.3 J/mol-K)/1000ΔG = -2032.6 kJ/mol. Therefore, the maximum work that can be obtained by burning 1.003 mol of C2H5OH is 2032.6 kJ/mol, which is the value of ΔG.
To know more about Gibbs free energy visit
https://brainly.com/question/13795204
#SPJ11
What is the answer for this question

Answers
Precipitate X is yellow while precipitate Y is white
What is the color of Lead II iodide and Lead II sulfate?The color of a lead precipitate can depend on a variety of factors, including the chemical composition of the precipitate, the size and shape of the particles, and the lighting conditions under which it is viewed. In the case of lead(II) iodide, the yellow color is due to the absorption of light in the visible spectrum by the compound's molecular structure.
The specific arrangement of atoms in the lead(II) iodide molecule causes it to absorb blue light, leaving only yellow light to be transmitted or reflected, which gives the compound its yellow color.
Learn more about precipitate:https://brainly.com/question/30904755
#SPJ1
how many more hydrogen atoms does a cyclohexane molecule have than a benzene molecule?
Answers
Six more hydrogen atoms does a cyclohexane molecule have than a benzene molecule.
Benzene (C₆H₆) is a cyclic hydrocarbon consisting of a hexagonal ring of carbon atoms with alternating single and double bonds. Each carbon atom in the benzene ring is bonded to one hydrogen atom. Therefore, a benzene molecule contains six carbon atoms and six hydrogen atoms.
Therefore, benzene contains six carbon atoms and six hydrogen atoms.
Cyclohexane (C₆H₁₂), on the other hand, is a saturated hydrocarbon known as a cycloalkane. It also consists of a hexagonal ring of carbon atoms, but unlike benzene, all the carbon-carbon bonds in cyclohexane are single bonds. This means that each carbon atom in the cyclohexane ring is bonded to two additional hydrogen atoms compared to benzene
Therefore, cyclohexane contains six carbon atoms and 12 hydrogen atoms.
The difference in the number of hydrogen atoms between a cyclohexane molecule and a benzene molecule is 12 - 6 = 6. Hence, a cyclohexane molecule has six more hydrogen atoms than a benzene molecule.
To know more about cyclohexane here
https://brainly.com/question/32069412
#SPJ4
Many nuclides with atomic numbers greater than 83 decay by processes such a electron emission. Explain the observation that the emissions from these unstable nuclides also ormally include alpha particles.
Answers
Answer:
The Explanation is described below:-
Explanation:
The purpose of the emission of an element with an atomic number higher than 83 mostly during the disintegration of the nucleus is that if the alpha particle is released, the recently established nuclei or the daughter would be closer to the stability band.
In other terms, in the case of an alpha particle explosion, the daughter nuclei would be more stable than the parent nuclei.
\(\alpha\) particles: Such particles are called alpha. They are basically divalent helium cations or helium nuclei with two protons and two neutrons. It is portrayed by
\(^4_2He\\\\or\\\\^4_2\alpha\)
The Stability band is the area in the graph that is plotted between the number of protons and the number of protons representing the stable nucleus.
Which of the following is an extensive property of a sample of aluminum?
Answers
Answer:
Entropy,mass and volume.... I think
Mass is the extensive property of a sample of aluminum. Therefore, option (A) is correct.
What is the extensive property of matter?The extensive property can be described as a physical property of matter that changes with a change in the size and shape of the matter. Therefore, the extensive property of any substance varies directly with the mass.
Extensive properties are the value of the property of the system that must be equal to the sum of the values of different parts of the system. These properties depend on the amount of matter contained in the system.
Examples of Extensive properties are temperature, pressure, density, boiling point, etc. The ratio of the two extensive properties is an intensive property such as density.
The mass of a sample of aluminum is an example of extensive property as it depends on the amount of aluminum in the given sample. This property of the sample is proportional to the size of the system.
Learn more about the extensive property, here:
https://brainly.com/question/13733851
#SPJ6
Your question is incomplete, most probably the complete question was,
Which of the following is an extensive property of a sample of aluminum?
A) Mass
B) Temperature
C) Pressure
D) Density
In order, what are the correct coefficients for the following equation?
___N2 + ___B → ___B3N2
Responses
1,3,1
1,1,3
3,1,1
1,3,3
Answers
The balanced chemical equation with coefficients is N₂+3 B → B₃N₂ which follows law of conservation of mass.
What is chemical equation?
Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ1
A rectangular tile, 15 by 18 inches, can be converted into square meters by which one of the following conversion setups?
A. (15 in × 18 in)(2.54 cm/1in)(1 m/100 cm)
B. (15 in × 18 in)(2.54 cm/1in)2(1 m/100 cm)
C. (15 in × 18 in)(2.54 cm/1 in)2(1 m/100 cm)2
D. (15 in × 18 in)(2.54 cm/1 in)(1 m/100 cm)2
Answers
Therefore, the correct conversion setup is:
C. (15 in × 18 in)(2.54 cm/1 in)2(1 m/100 cm)²
To convert the area of a rectangular tile from square inches to square meters, we need to use appropriate conversion factors.
Given:
Length = 15 inches
Width = 18 inches
To convert inches to centimeters, we use the conversion factor: 1 inch = 2.54 cm.
Now, let's consider the options:
A. (15 in × 18 in)(2.54 cm/1 in)(1 m/100 cm)
This option converts each side of the rectangular tile to centimeters and then to meters. However, since we want to find the area, we need to square the conversion factor. Therefore, option A is incorrect.
B. (15 in × 18 in)(2.54 cm/1 in)2(1 m/100 cm)
This option squares the conversion factor for inches to centimeters, but it doesn't square the conversion factor from centimeters to meters. Therefore, option B is also incorrect.
C. (15 in × 18 in)(2.54 cm/1 in)2(1 m/100 cm)2
This option squares both conversion factors correctly. It converts inches to centimeters and then centimeters to meters, while considering the area. Therefore, option C is the correct conversion setup.
D. (15 in × 18 in)(2.54 cm/1 in)(1 m/100 cm)2
This option squares the conversion factor from centimeters to meters but doesn't square the conversion factor from inches to centimeters. Therefore, option D is incorrect.
Therefore, the correct conversion setup is:
C. (15 in × 18 in)(2.54 cm/1 in)2(1 m/100 cm)2
Using this conversion setup will allow you to convert the area of the rectangular tile from square inches to square meters.
To know more about conversion factor:
https://brainly.com/question/33321070
#SPJ4
Which is an example of heterogeneous mixture?
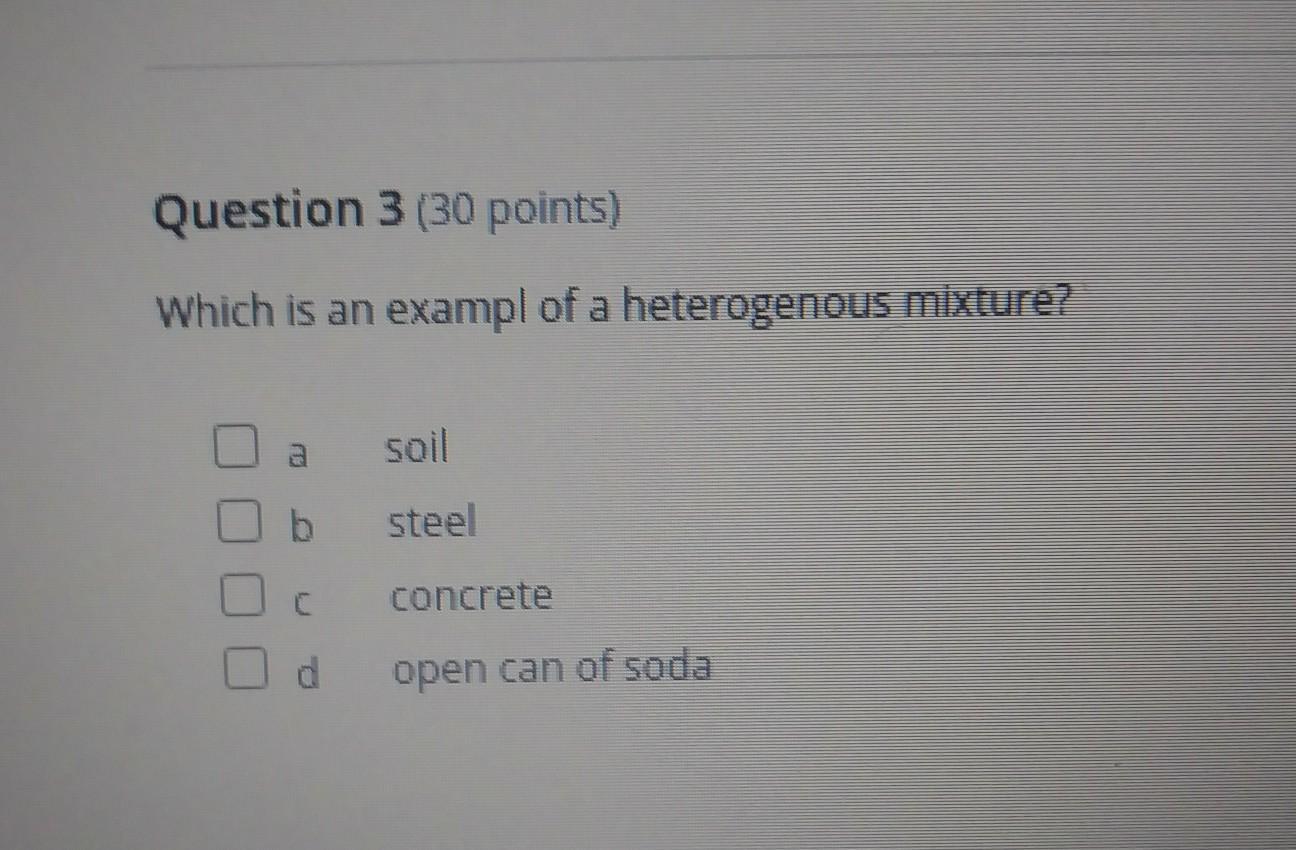
Answers
Answer:
soil
Explanation:
HELP CHEM ASAP PLZZZ!!!! Brainlist

Answers
Answer:
True.
Explanation:
The higher the 'Mol Concentration', the stronger the acid or base the substance is. For example...
1 Mol of HCl is less concentrated than 6 Mol HCl.
This is the same with bases:
1 Mol of NaOH is less concentrated than 6 Mol NaOH.