what is the ph of 0.027m of hclo4? round your answer to two decimal places.
Answers
The pH of 0.027 M HClO₄ is 1.57.
HClO₄ is a strong acid, meaning it completely dissociates in water. The chemical equation for the dissociation of HClO₄ is HClO₄ + H₋O → H₃O+ + ClO₄⁻. This means that all of the HClO₄ will become H₃O⁺ ions and contribute to the acidity of the solution.
To find the pH of the solution, we need to use the formula pH = -log[H₃O⁺]. Plugging in the concentration of H3O+ (which is equal to the concentration of HClO4 because it completely dissociates) gives us pH = -log(0.027) = 1.57.
The pH of 0.027 M HClO4 is 1.57. This is a very acidic solution due to the strong dissociation of the acid.
A pH of 1.57 is a very acidic solution. The pH scale ranges from 0 to 14, with a pH of 7 being neutral. Anything below 7 is considered acidic, and anything above 7 is considered basic. A pH of 1.57 indicates that the solution is strongly acidic due to the complete dissociation of the HClO₄.
In conclusion, the pH of 0.027 M HClO₄ is 1.57. This is a very acidic solution due to the strong dissociation of the acid.
To know more about pH, visit:
https://brainly.com/question/2288405
#SPJ11
Related Questions
How many moles of helium gas are present in 120 liters of STP
Answers
Yucca mountain, nevada is a potentially viable site for nuclear waste disposal because:________
Answers
Yucca Mountain, Nevada is a potentially viable site for nuclear waste disposal because it is an arid desert with very less precipitation and minimal contamination due to the nuclear waste.
Yucca Mountain, Nevada is a wide stretch of 160 km of dry desert with very little rainfall. This area is mainly composed of dense volcanic rock which contains minute pores which make the process of rainwater infiltration very slow and restricting.
Also, the nuclear waste will be stored in such a way that it is far above the water bodies of the mountain. This way the threat of water contamination is also out of question.
These all factors would effectively protect the waste and prevent the leak of harmful radioactive radiation from the disposal storage.
That being the case, Yucca Mountain, Nevada is a potentially viable site for nuclear waste disposal.
LEARN MORE ABOUT NUCLEAR WASTE HERE: https://brainly.com/question/4449362
#SPJ4
What is the optimum pH of formic acid-formate buffer? (The pK, of formic acid is 3.75.)
1.875
3.75
5.625
7.50
Answers
The optimum pH of formic acid - formate buffer is 3.75
What is Buffer ?
A substance or a solution which resists any changes in pH, when acid or alkali is added to it.
pH = pKa + log[base] / [acid]
Considering equimolar concentration of acid and base
pH = 3.75 + log(x)/(x)
pH = 3.75 + log (1)
pH = 3.75 + 0
pH = 3.75
Hence,
The optimum pH of formic acid - formate buffer is 3.75
Learn more about pH here ;
https://brainly.com/question/491373
#SPJ1
The particles in.......... can be separated from
heterogeneous mixtures by passing the mixture through a filter.
-suspension
-solution
-colloid
-pure substance
Answers
The particles in suspension can be separated from heterogenous mixtures by passing the mixture through a filter. Option 1.
Particles in a suspensionThe particles in suspension can be separated from heterogeneous mixtures by passing the mixture through a filter.
A suspension is a heterogeneous mixture in which the particles are large enough to settle out over time and can be separated by physical means such as filtration.
Other options such as solution, colloid, and pure substances cannot be separated using a filter.
More on suspensions can be found here: https://brainly.com/question/17650174
#SPJ1
Please help grades due today, I need 4-10

Answers
1) the contour interval on the map is 50
2) the letter that represents the highest point on the map is D
3) The landform represtented by letter B is a Hill.
4) the direction of the river at point A is flowing Downhill to the west toward G
5) The V-shaped contour lines represent valleys. They could also be ravines or gullies.
6) The letters that represent the two hills are: B and D
7) The letter that represents a steep slope is E
8) the letter that represents a gentle slope is A
9) The feature that is represented by the letter G on the map is a river.
10) The elevation change between positions F and C cannot be computed due to unclear markers. See below for how to compute elevation change.
Note that "rise over run" is an easy-to-remember equation for calculating a change in elevation as a decimal, which means the rise (the change in vertical distance) divided by the run (the change in horizontal distance).
Here's an example of how to use this formula:
Assume you have a map with contour lines spaced at 35-meter intervals and wish to calculate the elevation of a place 10 meters above a contour line with an elevation of 100 meters. The height of the point would be determined using the formula above as follows:
(35 * 10) + 100 = 350 + 100 = 450 meters
Learn more about Elevation Change:
https://brainly.com/question/29986657
#SPJ1
A gas is confined in a cylinder fitted with a movable piston. At 27ºC, the gas occupies a volume of 2.0 L under a pressure of 3.0 atm. The gas is heated to 47ºC and compressed to 5.0 atm. What volume does the gas occupy in its final state?
A: 0.78 L
B:1.3 L
C:2.1 L
D:0.48 L
Answers
This might help:

The volume of the gas is 1.27 L at a temperature is 47°C and the pressure is 5.0 atm. Therefore, option (B) is correct.
What is the ideal gas equation?The ideal gas equation is described as the equation of the state of a perfect gas. The ideal gas equation is the product of the pressure and volume of one-mole gas is equal to the multiplication of the absolute temperature and gas constant.
The mathematical equation for a perfect gas is as follows:
PV = nRT
where n is the moles of an ideal gas, P is the pressure of the gas, V is the volume, and R is the universal gas constant.
Given, the initial volume of the gas, V₁ = 2.0 L
The initial temperature of the gas, T₁ = 27° C = 300.15 K
The initial pressure of the gas, P₁ = 3.0 atm
The final temperature of the gas, T₂ = 47° C = 320.15 K
The initial pressure of the gas, P₂ = 5.0 atm
We know that for an ideal gas:
\(\frac{P_1V_1}{T_1} =\frac{P_2V_2}{T_2}\)
\(V_2=\frac{P_1V_1T_2}{T_1P_2}\)
\(V_2= \frac{3.0\times2.0\times320.15}{5.0\times 300.15}\)
\(V_2= 1.27 L\)
Therefore, the volume of the gas in its final state is equal to 1.27 L when the gas is heated to 47ºC and compressed to 5.0 atm.
Learn more about ideal gas equation, here:
brainly.com/question/3637553
#SPJ2
Calculate the number of molecules in 12.5 mol of CaCO3
Answers
The number of molecules in 12.5 moles of CaCO₃ is 7.525×10²⁴ molecules
How do I determine the number of molecules?From Avogadro's hypothesis, we understood that
1 mole of CaCO₃ = 6.02×10²³ molecules
Using the above information, we can obtain the number of molecules in 12.5 moles of CaCO₃ as illustrated below:
From Avogadro's hypothesis,
1 mole of CaCO₃ = 6.02×10²³ molecules
Therefore,
12.5 moles of CaCO₃ = (12.5 moles × 6.02×10²³ molecules) / 1 mole
12.5 moles of CaCO₃ = 7.525×10²⁴ molecules
Thus, we can conclude from the calculation made above that the number of molecules is 7.525×10²⁴ molecules
Learn more number of molecules:
https://brainly.com/question/20572558
#SPJ1
Which one of the following statements is correct about the reaction below? Mg(s) +2 HCl(aq) MgCl(s) + H2(g) A) Mg is the oxidizing agent because it is losing electrons. B) H is the reducing agent because it loses electrons. C) Cl is the reducing agent because it is an anion. D) H is the oxidizing agent because it gains electrons.
Answers
In the given reaction: Mg(s) + 2 HCl(aq) → MgCl(s) + H2(g) The correct statement about the reaction is: B) H is the reducing agent because it loses electrons.
Let's break down the given reaction and analyze the oxidation and reduction processes involved.
The reaction is: Mg(s) + 2 HCl(aq) → MgCl(s) + H2(g)
In this reaction, magnesium (Mg) reacts with hydrochloric acid (HCl) to produce magnesium chloride (MgCl) and hydrogen gas (H2).
To determine the oxidizing and reducing agents, we need to identify the species undergoing oxidation and reduction by looking at the changes in their oxidation states.
Oxidation involves an increase in oxidation state, while reduction involves a decrease in oxidation state.
Let's examine the oxidation states of the relevant elements:
Magnesium (Mg) in its elemental state has an oxidation state of 0.Hydrogen (H) in its elemental state has an oxidation state of 0.In hydrochloric acid (HCl), hydrogen (H) has an oxidation state of +1, and chlorine (Cl) has an oxidation state of -1.Now, let's analyze the reaction:
Mg(s) + 2 HCl(aq) → MgCl(s) + H2(g)
Magnesium (Mg) is being oxidized. Its oxidation state changes from 0 to +2 in MgCl. This indicates that magnesium is losing two electrons.Hydrogen (H) is being reduced. Its oxidation state changes from +1 in HCl to 0 in H2. This indicates that hydrogen is gaining one electron.Based on these observations, we can conclude the following:
Magnesium (Mg) is the reducing agent because it is losing electrons (undergoing oxidation).Hydrogen (H) is the oxidizing agent because it is gaining electrons (undergoing reduction).Therefore, the correct statement is:
B) H is the reducing agent because it loses electrons.
To learn more about oxidation and reduction, Visit:
https://brainly.com/question/13892498
#SPJ11
12
How many electrons shells would an atom of Sulfur have?Locate on your Periodic Table*
(5 Points)
03
6
16
O 32
13
Answers
What exacerbates the heat waves?
Answers
Answer:
A heatwave occurs when a system of high atmospheric pressure moves into an area and lasts two or more days.
Explanation:
In such a high-pressure system, air from upper levels of our atmosphere is pulled toward the ground, where it becomes compressed and increases in temperature.
Other than carbon being relatively small, what is another reason that carbon can form so many compounds? the ability to form four covalent bonds the ability to change shape the ability to form a diatomic molecule the ability to split its electrons
Answers
Answer:
Ability to form four covalent bonds.
Explanation:
Carbon is the first member of group 14. It is essentially a nonmetal. It is a small atom which regularly exhibits tetra valency. This means that carbon is able to form four covalent bonds to four chemical species which may be the same or different each time. This leaves room for many different possible combination patterns of carbon with other chemical species.
Hence carbon forms a very large number of compounds due to its small size and its ability for form four covalent bonds to other chemical species in any bonding situation.
Answer: the ability to form four covalent bonds
Explanation:
suppose that you add 25.6 g of an unknown molecular compound to 0.250 kg of benzene, which has a k f of 5.12 oc/m. with the added solute, you find that there is a freezing point depression of 3.54 oc compared to pure benzene. what is the molar mass (in g/mol) of the unknown compound?
Answers
If we add 25.6 g of an unknown molecular compound to the 0.250 kg of benzene, the molar mass of the unknown compound is 148.8 g/mol.
The Molality of the compound is given as :
ΔT = i Kf m
Where,
ΔT = freezing point depression = 3.54 °C
i = Van't Hoff factor of the Benzene = 1
Kf = constant of the freezing = 5.12 °C/m
m = molality = ?
m = ΔT / i Kf
m = 3.54 / 1 × 5.12
m = 0.69 mol
molality = moles / mass of benzene
moles = 0.172
The molar mass = mass / moles
The molar mass = 25.6 / 0.172
The molar mass = 148.8 g/mol
To learn more about molar mass here
https://brainly.com/question/16928753
#SPJ4
witch one is bigger 25yd or 75ft
Answers
Answer: in 25 yards there is 75 feet. Witch is the same to say that 25 yards is 75 feet.
Explanation:
Answer:
25yd is bigger
Explanation:
I need help finding the answer

Answers
The ion has 28 electrons, 31 protons, and 40 number of neutrons. Hence, option C is correct.
To determine the number of electrons, protons, and neutrons in the ion 71 31 Ga^3+, we can break down the information provided:
The symbol "Ga" represents the element Gallium, which has an atomic number of 31. This means that in a neutral atom of Gallium, the number of protons is 31.
The superscript "+3" indicates that the ion has a charge of +3. This means that the ion has lost 3 electrons compared to a neutral atom, resulting in a net positive charge.
To find the number of electrons in the ion, we subtract the charge (3) from the number of protons (31):
Number of electrons = Number of protons - Charge
= 31 - 3
= 28
Therefore, the ion 71 31 Ga^3+ has 28 electrons, 31 protons, and the number of neutrons can be determined by subtracting the number of protons from the atomic mass (71 - 31 = 40 neutrons).
For more question on neutrons click on
https://brainly.com/question/26952570
#SPJ11
The more variation in a species, the less likely the chance that species has of surviving. true or false
Answers
Answer:
The more variation in a species, the less likely the chance that species has of surviving. false
Explanation:
As species have heritable variation the species produce more offsprings that can survive and offsprings within a favorable variation are more likely to survive and reproduce. Hence favorable variation makes the species diverse and more in population. Thus greater are the chances of survival, a decrease in diversity will lead to a decline in variability, and fewer chances of survival.CO2 in beer is increased after fermentation by two different methods, what are they
Answers
The two main methods used to increase CO2 levels in beer after fermentation are natural carbonation and forced carbonation.
Natural carbonation involves adding a small amount of sugar to the beer before bottling or kegging. The residual yeast in the beer will consume the sugar, producing CO2 as a byproduct, which will dissolve into the beer, naturally carbonating it. This process can take anywhere from a few days to a few weeks, depending on the beer style and temperature.
Forced carbonation, on the other hand, involves using a CO2 tank to directly inject carbon dioxide into the beer. The beer is placed in a closed vessel and pressurized with CO2 until the desired level of carbonation is reached. This method is much quicker and more precise than natural carbonation, but it requires specialized equipment and can be more expensive.
Both methods have their advantages and disadvantages, and many breweries use a combination of both to achieve the desired level of carbonation for their beers. The level of carbonation can greatly affect the taste and mouthfeel of the beer, so it is an important consideration for brewers to get right.
For more such questions on fermentation, click on:
https://brainly.com/question/1411731
#SPJ11
In the 18th century, Italian biologist Lazzaro Spallanzani designed an experiment supporting the hypothesis that gastric juices are partly responsible for digesting food. Which human illness did Spallanzani's work eventually shed light on? intestinal ulcers mouth ulcers stomach ulcers skin ulcers
Answers
Stomach ulcers, I've read it online and I take the test
In the 18th century, Italian biologist Lazzaro Spallanzani designed an experiment supporting the hypothesis that gastric juices are partly responsible for digesting food. The human illness which Spallanzani's work eventually shed light on Stomach ulcers.
What is pH ?
pH is used to measure whether the substance is acidic, basic or neutral and the range is 0 - 14.
What is Gastric acid ?Gastric acid is also called gastric juice or stomach acid it is made up of water, enzymes, hydrochloric acid, electrolytes. The pH of gastric acid is between 1 and 3 that means it is acidic is nature.
Thus from the above conclusion we can say that In the 18th century, Italian biologist Lazzaro Spallanzani designed an experiment supporting the hypothesis that gastric juices are partly responsible for digesting food. The human illness which Spallanzani's work eventually shed light on Stomach ulcers.
Learn more about the pH here: https://brainly.com/question/24595796
#SPJ2
Imagine that both of these reactions are occurring at the same time. what is the net result of the reactions catalyzed by hexokinase and glucose 6-phosphatase?
Answers
The net result of the reactions catalyzed by hexokinase and glucose 6-phosphatase depends on the relative rates of the two reactions and the compartmentalization of the enzymes. If hexokinase dominates, glucose is converted to glucose 6-phosphate for energy production. If glucose 6-phosphatase dominates, glucose 6-phosphate is converted back to glucose for release into the bloodstream to maintain blood glucose levels.
The reactions catalyzed by hexokinase and glucose 6-phosphatase are essential steps in glucose metabolism, but they occur in different compartments of the cell and have opposing effects. Hexokinase catalyzes the conversion of glucose to glucose 6-phosphate in the cytoplasm, while glucose 6-phosphatase catalyzes the reverse reaction, converting glucose 6-phosphate back to glucose in the endoplasmic reticulum (ER) or the liver.
When both reactions occur simultaneously, the net result depends on the relative rates of the two reactions and the compartmentalization of the enzymes. If hexokinase activity dominates, glucose will be rapidly converted to glucose 6-phosphate in the cytoplasm. This is an important step in glycolysis, as glucose 6-phosphate can be further metabolized to produce energy.
On the other hand, if glucose 6-phosphatase activity dominates, glucose 6-phosphate will be dephosphorylated to glucose in the ER or the liver. This is crucial for the regulation of blood glucose levels, as it allows glucose to be released into the bloodstream for use by other tissues.
Learn more about phosphatase dominates
https://brainly.com/question/13050583
#SPJ11
Where are most volcanoes located? (Use information from the map.)
What is happening to the earth’s crust in these locations?
Answers
Calculate the pH after 0.13 mol NaOH are added to 1.00 L of 0.50M HC2H3O2 and 0.80M NaC2H3O2 buffer solution, Ka= 1.3 x 10^-5. I really need help, I'm struggling with this topic.
Answers
Determine how many mL of solution A (acetic acid-indicator solution) must be added to solution B (sodium acetate-indicator solution) to obtain a buffer solution that is equimolar in acetate and acetic acid. Solution A: 10.0 mL 3.0e-4M bromescol green
hoped I helped
Select all the names that could be used to describe the figure
A parallelogram
B quadrilateral
c rhombus
D square
1017 Answered
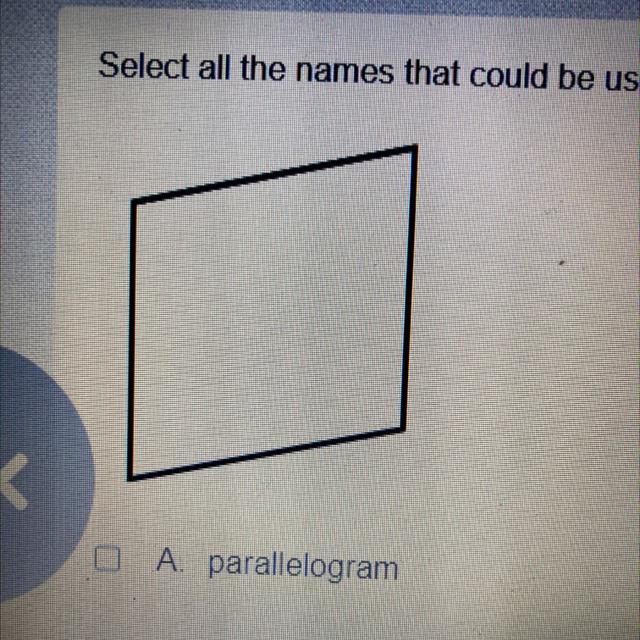
Answers
Answer:
Quadrilateral because there is no parallel and equal
select the correct options please
Which of the following compounds are not true organometallic compounds in the eyes of purists? Select one: A. Compounds 2 and 4 B. Compounds 1 and 5 C. Compound 5 only D. Compound 3 only E. Compound 2
Answers
To determine which compounds are not true organometallic compounds in the eyes of purists, we need to consider the definition of organometallic compounds.
Organometallic compounds are compounds that contain a direct bond between a carbon atom and a metal atom. Based on this definition, we can evaluate each compound provided:
Compound 1: This compound contains a direct bond between a carbon atom and a metal atom (M), so it is a true organometallic compound.
Compound 2: This compound contains a direct bond between a carbon atom and a metal atom (M), so it is a true organometallic compound.
Compound 3: This compound does not contain a direct bond between a carbon atom and a metal atom. Instead, it has a metal atom (M) coordinated to a ligand (L) without a direct carbon-metal bond. Therefore, it is not considered a true organometallic compound in the eyes of purists.
Compound 4: This compound contains a direct bond between a carbon atom and a metal atom (M), so it is a true organometallic compound.
Compound 5: This compound does not contain a direct bond between a carbon atom and a metal atom. It has a metal atom (M) coordinated to a ligand (L) without a direct carbon-metal bond. Therefore, it is not considered a true organometallic compound in the eyes of purists.
Based on the above analysis, the correct answer is:
D. Compound 3 only
Compound 3 is not considered a true organometallic compound since it lacks a direct carbon-metal bond.
To know more about bonds , visit;
https://brainly.com/question/25965295
#SPJ11
As a result of the reaction of 109.5 grams of a solution of hydrochloric acid with an excess of zinc, a gas with a volume of 3.36 litres Was released. Calculate the mass fraction of hydrochloric acid in the original solution
Answers
Explanation:
Volume of gas produced = 3.36 L
as molar volume of gas at STP is 22.4 L
so
no of moles of H2 = 3.36 ÷ 22.4 = 0.15 mole
from balanced reaction equation
Zn + 2HCl = ZnCl2 + H2
Ratio of no of moles between H2 : HCl is 1:2
as H2 moles = 0.15 mole
so
HCl moles reacted = 0.15 × 2 = 0.3 mole
as HCl molar mass = 1 + 35.5 = 36.5 g/mole
so Mass of 0.3 mole of HCl = 0.3 × 36.5 = 10.85 gram
fraction of HCl in Solution
= 10.85 ÷ 109.5 = 0.1
0.1 ×100 so HCl is 10% of solution mass
According to the concept of entropy, what will likely happen to most molecules over time?.
Answers
They're going to fall apart.
What most molecules will likely experience over time?A system's entropy cannot decrease unless heat is being lost from it. This indicates that entropy cannot decrease in a closed system (i.e., nothing enters or leaves, and that means nothing at all), so it must either remain constant or rise. Entropy can decrease, rise, or stay constant in an open system when movement between the system and its surroundings is conceivable. Both of these outcomes leave open the option of a constant state.
Entropy:-
The quantity of atom-alignment combinations that can exist in a system is known as entropy. The quantity of energy that cannot be used for work can also be calculated from an object's entropy.
Learn more about Entropy here:-
https://brainly.com/question/17241209
#SPJ4
pls help asap!!!
A 10 g piece of metal at 100°C is dropped into 10 mL (10 g) of water that is 20°C.
The final temperature of both the water and metal is 35°C. Which substance, the
metal or the water, has the highest specific heat? Explain why.
Answers
The metal has higher specific heat capacity than water because specific heat capacity is always positive & negative value of c(water) indicates that water can have a negative specific heat capacity.
What is the specific heat?The amount of heat required to increase the temperature of 1 gram of a substance by 1 degree Celsius (°C) is known as specific heat.
According to formula
q = m x c x ΔT
where q amount of heat absorbed or released, m mass of the substance, c specific heat capacity of the substance, and ΔT change in temperature.
We can start by finding the amount of heat released by the metal:
q(metal) = m x c(metal) x ΔT(metal)
q(metal)= 10 g x c(metal)x (100°C - 35°C)
q(metal)= 650 g°C x c(metal)
We can also find the amount of heat absorbed by the water:
q(water) = m x c(water) x ΔT(water)
q(water)= 10 g x c(water) x (35°C - 20°C)
q(water)= 150 g°C x c(water)
Since the metal releases heat and the water absorbs heat, we know that q(metal) = -q(water) (i.e., the heat lost by the metal is gained by the water).
Therefore:
650 g°C x c(metal) = -150 g°C x c(water)
Solving for c(water), we get:
c(water) = -650/150 x c(metal)
c(water) = -4.33 x c(metal)
Since specific heat capacity is always positive, we know that c(water) is negative in this case. This indicates that water cannot have a negative specific heat capacity. Therefore, the metal has a higher specific heat capacity than water.
To know more about specific heat visit :
https://brainly.com/question/11297584
#SPJ1
According to the electron sea model, the melting points of metals are not as extreme as the boiling points. This is because the cations and electrons are - in a metal. It does not take much energy for a solid to become liquid. But metallic bonds are very - , so it does require a lot of energy to separate atoms from the cations in their sea of electrons.
Answers
According to the electron sea model, the melting points of metals are not as extreme as the boiling points. Therefore, the given statement is correct is true.
What is electron sea model?The Electron Sea Model's whole hypothesis relies around the behavior of atoms throughout this bonding. The movement of unpaired electrons between positively charged metal ions in a mesh is known as metallic bonding.
According to the electron sea model, the melting points of metals are not as extreme as the boiling points. This is because the cations and electrons are - in a metal. It does not take much energy for a solid to become liquid. But metallic bonds are very - , so it does require a lot of energy to separate atoms from the cations in their sea of electrons. This statement is true.
Therefore, the given statement is correct is true.
To know more about electron sea model, here:
https://brainly.com/question/30003484
#SPJ1
in 25.0 g of a 7.50% by mass solution of caso4: a) how many grams of solute are present? b) how many grams of solvent are present?
Answers
a) Solute weight in grams: (25*7.5)/100 = 1.875 gm.
b) Because the weight of a solution is equal to the sum of the weights of the solute and solvent,
Solution weight is 100 grams.
Solvent weight in grams: 100 - 1.875 = 98.125 gm.
Given that water has a density of 1 g/ml, the formula to determine how much solute to add to a solution in weight percent is as follows: grams of solute = (wt% solution) x (ml of water) (100 - wt% solution). The ratio of a solute—a material that dissolves—to a solvent—a substance that does not dissolve—determines the concentration of a solution in chemistry. C = m/V, where C is the concentration, m is the mass of the solute dissolved, and V is the overall volume of the solution, is the accepted formula.
learn more about Solution here:
https://brainly.com/question/1416865
#SPJ4
Mark scheme
Remember how we work out relative formula mass: Mr = Sum of (Ar of element x number
of atoms in element)
Multiply the number of atoms in each element by the element's relative atomic mass and
add these up:
Mr (1 x 23) + (1 x 23) + (1x 16)
Work out the answer: Mr = 23 + 23 + 16
V
Mr= 62
Feedback?

Answers
The given relative mass formula is correct. The weight in grams of the number of atoms of an element contained in 12.00 g of carbon-12 is known as the relative atomic mass of the element.
What is mass relative to?The ratio of an element's average atomic mass to the unified atomic mass unit is the relative atomic mass, or Ar. The average mass of an element's isotopes is used to calculate the relative atomic mass.
What exactly are absolute and relative masses?Absolute mass is the total mass of all protons and neutrons, whereas relative mass is the average atomic mass of all the isotopes present in a given percentage. As an illustration, the average atomic mass of carbon, calculated using the proportions of the isotopes C-12, C-13, and C-14, is 12.01 while the absolute mass of carbon is 12.0 amu.
What is the atomic mass equation?An element's mass number is determined by the sum of its proton and neutron counts: Protons and neutrons together make up mass.
To know more about relative mass visit:
https://brainly.com/question/29963883
#SPJ1
which of the following options correctly describe a titration? select all that apply. multiple select question. in a titration the volumes of both solutions must be known. in a titration, the standard solution is one that has an accurately known concentration. a titration relies on a reaction that takes place in solution. a titration can only be used to determine the concentration of an acid or a base. the concentrations of both solutions must be known before the titration is carried out.
Answers
Statement correctly describes a titration : A titration relies on a reaction that takes place in solution.
What Is Meant by TitrationBasically, titration is a chemical method to determine the concentration of a solution. The trick is to react a solution in a certain volume with another solution whose concentration of the substance is known. This known solution is called a frozen solution. While the purpose of the titration itself is to determine the pH level of a chemical substance. The end point is when the indicator changes color.
This titration measurement usually uses several special tools, including a burette, stative, Erlenmeyer tube, rubber suction cup, watch glass, dropper pipette, measuring flask, and volume pipette. One of the conditions for the titration to run well is that it is characterized by a fast reaction, you can even use a catalyst to speed up the reaction. Furthermore, the reaction proceeds simply and the stoichiometric equation is clear. Then there are no side reactions that can affect the main reaction.
Titration TypeBased on the type, the titration is divided into four types. The three types are redox titrations, complexation titrations, and acid-base titrations and argentometry. For more details about these three types of titration, you can see in the following review.
Redox Titration
For this type of redox titration is a type of titration that processes with redox reactions. Redox in titration is still divided into three. Namely those that use I2 and are indirect titrations. This is because the reacted I2 is still made by the previous redox reaction. Meanwhile for the second type is iodometric titration which is used directly in I2 and can be called a direct reaction. The third type of redox is permanganometric where the reaction utilizes Mn2+ ions.
Complexation TitrationThis type of complexation titration is actually a titration that uses complexation reactions and the formation of complex ions. Its use is usually to analyze metal levels. When you want to do a type titration there are several things to consider. This is more because the formation of complex ions is very specific under certain conditions.
Acid Base TitrationThe third type of titration is the acid-base titration. Actually, for this titration it refers more to a quantitative analysis method based on acid-base reactions. The indicators used are usually those that can profile the color change at a certain pH.
Argentometry TitrationThis last type is argentometric titration. This titration is a titration commonly used for precipitation reactions. Based on the principle of argentometric titration regarding solubility as well as the product constants of the reacting reagents. The method for Argentometric titration is divided into the Mohr method, the Volhard method, and the Fajans method.
Learn more about titration at https://brainly.com/question/24704707.
#SPJ4
I need help with this ASAP pls

Answers
Answer:
2c 1s
Expination: it came by there are 2carbon and 1sulphur
Answer:
2c1s
Explanation:
2c1s make me brainlist