What is the solubility of potassium nitrate in 20 °c water.
Answers
The solubility of potassium nitrate (KNO₃) in water at 20 °C is approximately 13.3 g/100 mL.
Solubility of a substance is the maximum amount of solute that can be dissolved in a solvent at a specific temperature, and pressure. It is an important factor in understanding and predicting chemical reactions, phase transitions, and other physical processes. Potassium nitrate (KNO₃) is a water-soluble salt commonly used in the production of fertilizers, food preservatives, and fireworks.
The solubility of KNO₃ in water depends on temperature, pressure, and other factors. At 20°C, the solubility of KNO₃ in water is approximately 13.3 g/100 mL. This means that 13.3 grams of KNO₃ can be dissolved in 100 milliliters of water at 20°C. The solubility of KNO₃ in water increases with temperature.
Learn more about solubility here:
https://brainly.com/question/28170449
#SPJ11
Related Questions
please helppp this is about to be due
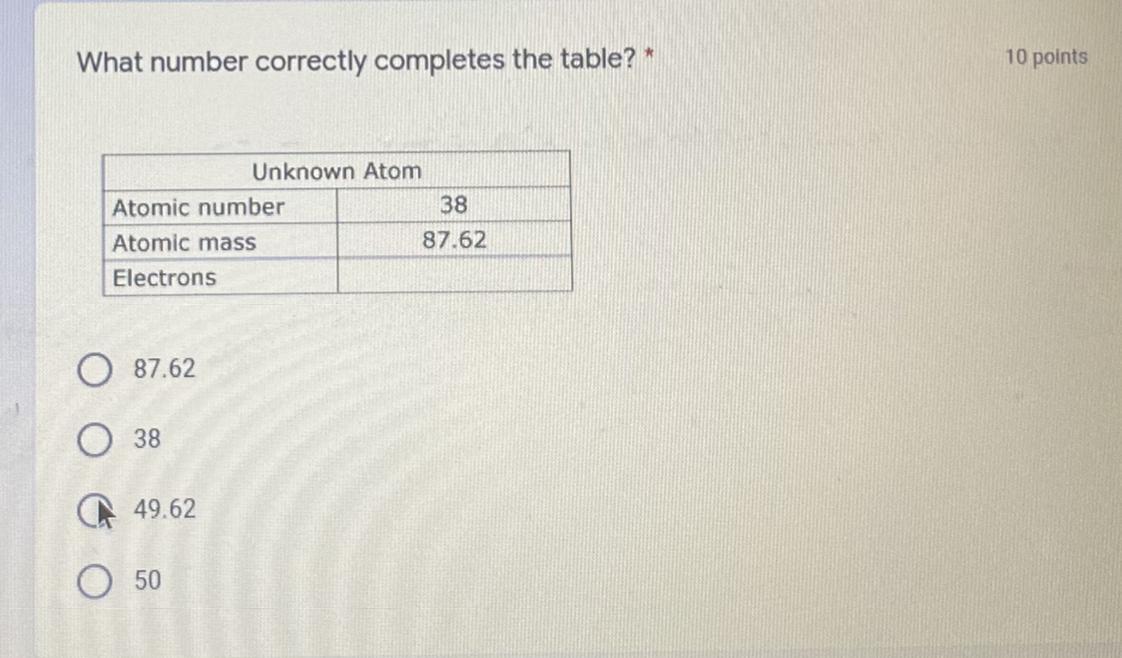
Answers
Answer:
38
Explanation:
An atom consist of electron, protons and neutrons. Protons and neutrons are present with in nucleus while the electrons are present out side the nucleus.
All these three subatomic particles construct an atom. A neutral atom have equal number of proton and electron. In other words we can say that negative and positive charges are equal in magnitude and cancel the each other. For example if neutral atom has 6 protons than it must have 6 electrons. The sum of neutrons and protons is the mass number of an atom while the number of protons are number of electrons is the atomic number of an atom.
In given question atomic number is 38 which means number of proton and number of electrons are also 38 because number of protons are electrons are always equal in neutral atom.
A 150.0 ml sample of an aqueous solution at 25°c contains 15.2 mg of an unknown nonelectrolyte compound. if the solution has an osmotic pressure of 8.44 torr, what is the molar mass of the unknown compound?
Answers
The molecular mass of the unknown compound found in the aqueous solution was 223.2g/mol
The sum of the atomic masses of all atoms in a molecule is called as molecular weight
So to get molar mass of the compound from the given weight the concentration of compound is elucidated and thereby leading to the calculation of molar mass
Then the concentration of solute in an aqueous solution can be derived from the osmotic pressure equation
So the osmotic pressure = van't hoff factor then the molarity of solute(M) gas constant(R) temprature(T)
From the given values in the question and applying constant values
8.44 torr = 1(for non electrolytes)× M×62.3637L⁻¹torr⁻¹mol⁻¹×298K
M = 4.54×10⁻⁴M
From the molarity of the given compound the molare mass can be calculated as
Molarity = weight/ molecular weight × 1000/volume(ml)
4.54×10⁻⁴M = 0..0152g/molecular weight × 1000/150ml)
Molecular weight = 223.2g/mol
Know more about compound
https://brainly.com/question/14127248
#SPJ4
The compound NH³ contains two double covalent bonds.
(Never True, Always True, Sometimes True)
Answers
The compound NH3 contains two double covalent bonds. The given statement is never true because its single covalent bonds
NH3, also known as ammonia, consists of one nitrogen atom (N) and three hydrogen atoms (H). In this compound, the nitrogen atom forms three single covalent bonds with the three hydrogen atoms. A covalent bond occurs when two atoms share a pair of electrons, and in ammonia, each hydrogen atom shares one electron with the nitrogen atom. There are no double covalent bonds in NH3, as double bonds would require two pairs of shared electrons between the same two atoms, which is not the case in this compound.
Ammonia has a trigonal pyramidal molecular geometry with the nitrogen atom at the center and the hydrogen atoms surrounding it. The nitrogen atom also has one lone pair of electrons, which contributes to its basic properties and the polar nature of the molecule. So, the correct answer to your question is that it is Never True that NH3 contains two double covalent bonds. The compound NH3 contains two double covalent bonds. The given statement is never true because its single covalent bonds
learn more about covalent bonds here:
https://brainly.com/question/8295784
#SPJ11
Consider the reaction X + Z ?
Assume the following:
1) The product is an ionic compound.
2) Element X has three valence electrons
3) Element Z needs two valence electrons to have completed s & p subshells (full octet)
What would make the most sense for the formula of the product?
Answers
Answer:
X2Z3
Explanation:
Based on the clues, X is a metal and Z is a non-metal.
X could be aluminum and Z would need to be an element from the oxygen family.
Can you show me the answer and explain?

Answers
Answer:
C
Explanation:
looking at a periodic table X is fluorine and Y is potassium
Fluorine is in group 7 and forms a 1- charge (which gains electrons) and potassium is in group 1 and forms a 1+ charge (which loses electrons)
Fluorine (X) has an electronic structure of 2,7 and needs to gain an electron from Potassium (Y) to have a full outer shell and potassium has an electronic structure of 2,8,8,1 so needs to lose an electron to have a full outer shell as well. This means that the electron that potassium (Y) has lost is given away to fluorine (X), so both elements become stable.
This is known as ionic bonding where metals (like potassium) lose electrons and non-metals (like fluorine) gain electrons to become more stable, forming ions
Any further clarification let me know
Cis- and trans-2-butene can both be hydrogenated to butane; thus their energies can be compared. the _____-isomer releases less energy upon hydrogenation therefore _____-2-butene higher in energy.
Answers
Cis- and trans-2-butene can both be hydrogenated to butane; thus their energies can be compared. the trans isomer releases less energy upon hydrogenation therefore cis-2-butene higher in energy.
What is hydrogenation?The process of addition of hydrogen atom to another compound in the presence of catalyst such as nickel, cobalt etc. is termed as hydrogenation process.
Why trans has lesser energy of hydrogenation?As in trans- isomer, similar atoms or group of atoms are on opposite side. Due to which there is less repulsion between the atoms which results in more stability of trans isomer. On the other hand in cis- isomer, similar atoms or group of atoms are on same side, due to which there is more repulsion between the atoms which results in less stability of cis-isomer.
Due to more stability of trans- isomer less energy is released to add hydrogen to the trans butene as compared to cis butene for the formation of butane.
Thus we concluded that the trans isomer releases less energy than cis isomer in hydrogenation process.
learn more about hydrogenation:
https://brainly.com/question/10150087
#SPJ4
Match the levels of Po2 and Pcou with the corresponding point in the circulatory route. Tissue Fluid Poz 40 mm Hg Pco2 46 mm Hg Po295 mm Hg Pco2 40 mm Hg Oxygenated Blood Poz 104 mm Hg PcO2 40 mm Hg Alveolar Air Reset
Answers
The levels of Po2 and Pco2 in the circulatory route are tightly regulated to ensure that oxygen is delivered to the tissues and carbon dioxide is removed from the body. This process involves the exchange of gases between the alveolar air, oxygenated blood, and tissue fluid, with each step corresponding to specific levels of Po2 and Pco2.
In the circulatory route, the oxygenated blood leaves the lungs and enters the arteries with a Po2 of 104 mm Hg and a Pco2 of 40 mm Hg. As it travels through the body, it delivers oxygen to the tissues and picks up carbon dioxide. As a result, the Po2 in the tissue fluid drops to 40 mm Hg, while the Pco2 rises to 46 mm Hg. This occurs because oxygen is being used up by the cells in the tissues, while carbon dioxide is being produced as a byproduct of cellular respiration.
Once the blood has picked up carbon dioxide and delivered oxygen to the tissues, it returns to the heart via the veins. The oxygenated blood then enters the lungs once again, where it unloads carbon dioxide and picks up oxygen from the alveolar air. At this point, the Po2 in the alveolar air is 95 mm Hg, while the Pco2 is 40 mm Hg. The oxygenated blood then leaves the lungs with a Po2 of 104 mm Hg and a Pco2 of 40 mm Hg, completing the circulatory route.
In summary, the levels of Po2 and Pco2 in the circulatory route are tightly regulated to ensure that oxygen is delivered to the tissues and carbon dioxide is removed from the body. This process involves the exchange of gases between the alveolar air, oxygenated blood, and tissue fluid, with each step corresponding to specific levels of Po2 and Pco2.
for more such question on circulatory route
https://brainly.com/question/23522081
#SPJ11
Which reaction is a neutralisation reaction?
Answers
Answer:
The interaction of H+ ions and OH- ions produces water in a neutralization reaction, which occurs when an acid and a base combine to make water and a salt.
Explanation:
An acid and a base combine to produce an ionic molecule and potentially water in a neutralization reaction.
Identify the reactants in the following equation.
Li + Cl2 ---> Lici
A) the entire equation is the reactant
B) Li + C12
C) Lici
D) the arrow
Answers
Answer:
Li + Cl₂
Explanation:
left side of the arrow are reactants and right side of the arrow are products.
A substance contains 36.1 percent calcium and 63.9 percent chlorine by weight. What is the empirical formula of the given compound?
A.
CaCl
B.
Ca0.9Cl1.8
C.
CaCl2
D.
Ca2Cl4
Answers
Answer:
C
Explanation:
Answer:C-CaCl2
Explanation:The answer is C because the chemical formula of calcium chloride is CaCl2.
Student a says that he can insert any value of optical density in the equation of standard curve to determine the corresponding value of protein content. does the student say it right or wrong? explain why?
Answers
The equation of the standard curve is created by plotting known concentrations of protein against their corresponding optical density values. Once the equation of the standard curve is established, any unknown optical density value can be inserted into the equation to determine the corresponding protein content. The statement is correct.
The student's statement is correct because the standard curve is created by plotting known concentrations of protein against their corresponding optical density values. The equation of the standard curve can then be used to determine the protein concentration of unknown samples based on their optical density. This is achieved by inserting the unknown optical density value into the equation of the standard curve to obtain the corresponding protein concentration. Therefore, the student's statement is right.
In conclusion, the student is correct. By using the equation of the standard curve, any value of optical density can be used to determine the corresponding value of protein content. It is essential to understand the principle behind creating a standard curve to get accurate results when determining protein content.
To know more about concentrations of protein visit:
brainly.com/question/29642358
#SPJ11
you are reading your aceable course and come across this: 475.021, f.s. what is this mysterious glyph trying to tell you?
Answers
475.021, f.s., refers to the Florida Statute (f.s.) for driver's license suspensions and revocations. This particular statute deals with the suspension of licenses for drivers who fail to pay court fines, costs, and fees.
The statute states that failure to pay the required court fees within 30 days will result in the suspension of the driver's license.
After 90 days, the suspension becomes permanent until the fees are paid. In summary, the mysterious glyph is trying to tell you about the legal statute in Florida regarding the suspension of driver's licenses for non-payment of court fees, fines, and costs.
To learn more about suspension, visit:
https://brainly.com/question/32696604
#SPJ11
how much heat does it take to increase the temperature of 1.80 molmol of an ideal gas by 40.0 kk near room temperature if the gas is held at constant volume and is diatomic?
Answers
The amount of heat required to increase the temperature of 1.80 mol of an ideal gas by 40.0 K near room temperature if the gas is held at constant volume and is diatomic is 1498.10 J.
Given that we need to determine the amount of heat required to increase the temperature of 1.80 mol of an ideal gas by 40.0 K near room temperature if the gas is held at constant volume and is diatomic. Let’s begin with the ideal gas equation PV = nRT, where P = Pressure , V = Volume (held constant)n = Number of moles, R = Universal gas constant = 8.31 J/mol K (given)T = Temperature (in Kelvin)We know that the gas is diatomic i.e. it consists of two atoms; hence the degree of freedom of the gas will be f = 5/2 (as per the rule for diatomic molecules).
We are to find out the amount of heat absorbed by the gas, which can be calculated using the First Law of Thermodynamics. It is given byΔU = q + w, whereΔU = Change in internal energy of the gas, q = Heat absorbed by the gas (what we need to determine)w = Work done by the gas (at constant volume, w = 0)The change in internal energy is given by the expressionΔU = (f/2) nR ΔTPutting the given values in the above expression, we get ΔU = (5/4) x 1.80 x 8.31 x 40.0= 1498.10 J
∴ The amount of heat required to increase the temperature of 1.80 mol of an ideal gas by 40.0 K near room temperature if the gas is held at constant volume and is diatomic is 1498.10 J.
To know more about amount of heat, refer
https://brainly.com/question/25603269
#SPJ11
To what volume should you dilute 25 mL of a 12.0 M H2SO4 solution to obtain a 0.170 M H2SO4 solution
Answers
1764.71mL will be obtained to make 0.170M \(H_2SO_4\)
To dilute 25 mL of a 12.0 M \(H_2SO_4\) solution to obtain a 0.170 M \(H_2SO_4\) solution, you should use the dilution formula:
M1V1 = M2V2
Where M1 and V1 are the initial molarity and volume, and M2 and V2 are the final molarity and volume, respectively. In this case:
M1 = 12.0 M
V1 = 25 mL
M2 = 0.170 M
Plug in the values and solve for V2:
(12.0 M)(25 mL) = (0.170 M)(V2)
300 = 0.170V2
V2 = 300 / 0.170 ≈ 1764.71 mL
So, you should dilute the 25 mL of 12.0 M H2SO4 solution to approximately 1764.71 mL to obtain a 0.170 M \(H_2SO_4\) solution.
To know more about dilution formulas:
https://brainly.com/question/7208939
#SPJ11
Nitrogen gas is the major component of air. a sample of nitrogen gas in a glass bulb weighs 243 mg. what is this mass in si base units of mass (kilograms)?
Answers
Nitrogen gas is the major component of air. a sample of nitrogen gas in a glass bulb weighs 243 mg. 2.43 × 10-4 kg is this mass in si base units of mass (kilograms).
Nitrogen, or N, using its scientific abbreviation, is a colorless, odorless element. Nitrogen is in the ground under our feet, in the water we drink, and in the air, we breathe. In fact, nitrogen is the most abundant element in the Earth's atmosphere, with approximately 78% of the atmosphere being composed of nitrogen.
Nitrogen is commonly used during sample preparation for chemical analysis. Used to concentrate and reduce the volume of liquid samples. Nitrogen is also important for the chemical industry. It is used in the production of fertilizers, nitric acid, nylon, dyes, and explosives*. Exposure to very high concentrations of pure nitrogen can cause dizziness, drowsiness, displacing oxygen from the air, unconsciousness, and death.
Learn more about nitrogen here
https://brainly.com/question/1380063
#SPJ4
Which would elute from a column first: a spherical or linear molecule?
Answers
This is because a spherical molecule has a more compact structure and therefore experiences less surface interaction with the stationary phase of the column, allowing it to move more easily through the column and elute first.
What is Linear Molecule?
A linear molecule is a molecule that has a straight or chain-like structure, where the atoms are arranged in a line or a chain. Linear molecules are characterized by having two or more atoms connected by single covalent bonds, and they may or may not have double or triple bonds as well.
In contrast, a linear molecule has a more extended structure and experiences more surface interactions with the stationary phase of the column, leading to slower movement through the column and later elution. However, it is important to note that elution order also depends on other factors such as the polarity of the stationary and mobile phases, the size and shape of the column, and the specific properties of the molecules being separated.
Learn more about Linear Molecule from the given link
https://brainly.com/question/29819053
#SPJ4
for 280.0 ml of pure water, calculate the initial ph and the final ph after adding 0.028 mol of naoh .
Answers
The initial pH of pure water is 7.0, and after adding 0.028 mol of NaOH to 280.0 ml of water, the final pH is approximately 13.0 due to an increase in hydroxide ion concentration.
The initial pH of pure water is 7.0, as it is considered neutral. After adding 0.028 mol of NaOH to 280.0 ml of pure water, the final pH can be calculated.
Pure water has a neutral pH of 7.0, which means it has an equal concentration of hydrogen ions (H+) and hydroxide ions (OH-). When NaOH is added to water, it dissociates into Na+ and OH- ions. The OH- ions react with the H+ ions in the water, resulting in an increase in the concentration of hydroxide ions and a decrease in the concentration of hydrogen ions.
To calculate the final pH, we need to determine the concentration of OH- ions after the addition of NaOH. Since 0.028 mol of NaOH is added to 280.0 ml of water, the concentration of OH- ions can be calculated using the molarity formula:
Molarity = Moles of solute / Volume of solution (in liters)
Converting the volume of water to liters (280.0 ml = 0.280 L), we can calculate the molarity of the OH- ions:
Molarity of OH- = (0.028 mol) / (0.280 L) = 0.10 M
The concentration of OH- ions corresponds to the pOH value, which is the negative logarithm (base 10) of the hydroxide ion concentration:
pOH = -log [OH-] = -log (0.10) ≈ 1.0
Since pH + pOH = 14 (for neutral solutions), the final pH can be calculated:
pH = 14 - pOH = 14 - 1.0 = 13.0
Therefore, the final pH after adding 0.028 mol of NaOH to 280.0 ml of pure water is approximately 13.0.
To learn more about Molarity click here: brainly.com/question/2817451
#SPJ11
IM GIVING BRAINLEST!
What is the name of Pb(NO3 ) 2 Explain how you determined the bond type and the steps you used to determine the naming convention for the compound
Answers
Name of Pb(NO3 )2 is Lead(II) nitrate.
Pb(NO3)2 is ionic as well as covalent
Pb is the symbol of lead and NO3 is a complex ion called nitrate. It has one nitrogen and three oxygens. Pb(NO3)2 is ionic as well as covalent. NO3 is covalent in nature because both nitrogen and oxygen are non-metals and therefore an ionic bond cannot be formed between them.
Ionic compounds are formed when a metal reacts with a nonmetal whereas covalent compounds are formed when two non -metals react with each other. Binary compounds containing hydrogen are usually covalent compounds as hydrogen is a non - metal.
An ionic bond donates an electron to the other atom in the bond whereas electrons in a covalent bond are shared equally between the atoms.
To know more about bond types, refer
https://brainly.com/question/2234173
#SPJ13
If an aluminum scuba tank contains compressed air at 2.899 x 103 psi, what is the pressure If an aluminum scuba tank contains compressed air at 2.899 x 103 psi, what is the pressure expressed in inches of mercury?expressed in inches of mercury?
Answers
The pressure in the aluminum scuba tank, containing compressed air at 2.899 x 103 psi, expressed in inches of mercury is approximately 5,908 inHg.
To convert the pressure from psi to inches of mercury, you can use the following conversion factor: 1 psi = 2.036 inches of mercury. Given that the aluminum scuba tank contains compressed air at 2.899 x 10³ psi, you can calculate the pressure in inches of mercury by multiplying the pressure in psi by the conversion factor:
Pressure in inches of mercury = (2.899 x 10³ psi) * (2.036 inHg/psi)
Pressure in inches of mercury ≈ 5,908 inHg
So, the pressure in the aluminum scuba tank expressed in inches of mercury is approximately 5,908 inHg.
More on pressure: https://brainly.com/question/31567902
#SPJ11
the third law of thermodynamics describes the entropy of a: select the correct answer below: solid liquid gas all of the above
Answers
The third law of thermodynamics describes the entropy of a: solid.
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius). This law implies that at absolute zero, a perfectly ordered and pure crystalline solid will have zero entropy.
The third law of thermodynamics is not specific to liquids or gases but applies to solids. In a solid, the molecules are highly ordered and have fixed positions in a regular lattice structure. As the temperature decreases towards absolute zero, the thermal motion of the molecules reduces, and the system becomes more ordered, resulting in a decrease in entropy.
In contrast, liquids and gases have higher entropy compared to solids at absolute zero because their molecules have more freedom of movement and are not as tightly arranged. Therefore, the third law of thermodynamics specifically addresses the entropy of solids and does not apply to liquids or gases.
To learn more about law of thermodynamics, here
https://brainly.com/question/1368306
#SPJ4
A clown is trying to lift a refrigerator of wigs a height of 2 meters. It would take him a force of 80
Newtons to lift the fridge without a simple machine.
a. How much work would be required to lift this
fridge of wigs without a machine? Don’t forget
a unit for your answer!
b. Assuming no friction, how much effort force would be required to lift the fridge of wigs with a lever that has a mechanical advantage of 4? Don’t forget a unit for your answer!
Answers
a. The work required to lift the fridge of wigs without a machine is 160 Joules. b. The effort force required to lift the fridge of wigs with a lever that has a mechanical advantage of 4 is one-fourth (1/4) of the weight of the fridge.
What is work?Work is defined as the energy transferred to or from an object by means of a force acting on the object, causing it to move in the direction of the force.
a. To lift the fridge of wigs without a machine, the clown would need to apply a force of 80 Newtons over a distance of 2 meters. The work done would be given by the formula:
Work = Force x Distance
Therefore, the work required would be:
Work = 80 N x 2 m = 160 Joules
So, the work required to lift the fridge of wigs without a machine is 160 Joules.
b. If the lever has a mechanical advantage of 4, it means that the effort force required would be one-fourth (1/4) of the weight of the fridge of wigs. Since the weight of the fridge is not given, let's assume it to be W Newtons.
According to the principle of the lever, the product of the effort force and its distance from the fulcrum is equal to the product of the load force (the weight of the fridge) and its distance from the fulcrum. Assuming that the effort force is applied at a distance of 0.5 meters from the fulcrum, we can write:
Effort force x 0.5 m = (1/4)W x 2 m
Simplifying the equation, we get:
Effort force = (1/4)W x 2 m / 0.5 m = (1/4)W x 4
Effort force = W Newtons / 4
Therefore, the effort force required to lift the fridge of wigs with a lever that has a mechanical advantage of 4 is one-fourth (1/4) of the weight of the fridge.
Learn more about work here:
https://brainly.com/question/18094932
#SPJ1
Please help me with number 12

Answers
Answer:
organisms in rain water and the number of organisms in pond water because the dependant variable never changes
Explanation:
because the dependant variable never changes
if i need to consume 20.0 grams of fructose (c6h12o6) each day, how many milliliters of juice, a solution with a fructose concentration of 0.500 m, should i drink?
Answers
C6H12O6 is the chemical formula for Glucose. If you need to consume 20.0 grams of (C6H12O6) each day, then you should drink 693.8 milliliters of juice.
What is Molarity ?The concentration of a solution is modeled using molarity. The number of moles of solute dissolved in one liter of a solution is its molarity. As a carbohydrate,
(C6H12O6) has a molar mass of 180.16 g/mol.
As a result, moles of (C6H12O6) are calculated as follows: 25g/180.16g/mol = 0.138765mol.
Thus, volume of (C6H12O6) equals moles of glucose/molarity equals 0.138765mol/0.20M = 0.6938 L = 693.8 ml, where 1000 ml equals 1 liter.
Thus, 693.8 ml of a (C6H12O6) solution containing 0.20 M are required.
To know more about Molarity visit: https://brainly.com/question/16727614
#SPJ4
which of the following are the main forms of chemical inputs and outputs that are recycled within an ecosystem?
Answers
The main forms of chemical inputs and outputs that are recycled within an ecosystem are Water, Carbon, Nitrogen, Phosphorus.
1. Water (H₂O): Water is a vital chemical component that cycles through ecosystems. It enters the ecosystem through various sources such as precipitation, surface runoff, or groundwater.
Water is utilized by organisms for various physiological processes, and it is released back into the environment through transpiration, evaporation, or as runoff.
2. Carbon (C): Carbon is a fundamental element present in organic compounds. It cycles through ecosystems in the form of carbon dioxide (CO₂), which is absorbed by plants during photosynthesis.
Through photosynthesis, plants convert CO₂ into organic compounds, which are consumed by other organisms. When organisms respire or decompose, carbon is released back into the atmosphere as CO₂
3. Nitrogen (N): Nitrogen is an essential nutrient for living organisms and is found in various forms in the ecosystem, such as nitrogen gas (N₂), ammonia (NH₃), nitrate (NO₃-), and organic nitrogen compounds.
Nitrogen enters the ecosystem through biological nitrogen fixation, industrial activities, or atmospheric deposition. Within the ecosystem, nitrogen is taken up by plants and incorporated into proteins and other organic molecules.
Through various processes like nitrogen fixation, nitrification, and denitrification, nitrogen is converted and recycled within the ecosystem.
4. Phosphorus (P): Phosphorus is another vital nutrient that cycles within ecosystems. It enters the ecosystem through the weathering of rocks and minerals.
Plants take up phosphorus from the soil and incorporate it into organic compounds. When plants and animals die, phosphorus is released through decomposition and becomes available again for uptake by plants.
To know more about ecosystem refer here:
https://brainly.com/question/9434120#
#SPJ11
In a science lab, Cash mixes two clear liquids together in a beaker. Bubbles are produced, and a white solid forms and settles to the bottom. Which statement below describes what happened? a A physical change occurred, a gas and precipitate was produced b A physical change occurred, only a gas was produced c A chemical change occurred, only a gas was produced d A chemical change occurred, a gas and precipitate was produced
Answers
Answer:
A chemical change occurred, a gas and precipitate was produced
Explanation:
From the question , we are informed of science lab, where Cash mixes two clear liquids together in a beaker. Bubbles are produced, and a white solid forms and settles to the bottom.
In this case the change that took place is chemical change ( is one where new product are formed after two substance react) the bubbles that is produced signify the presence of gas in the product, white solid formed is reffered to as a precipitate( which is reffered to as solid that is been formed from a particular solution).
The amount of kinetic energy an object has depends on its:
**hint: look up the equation for kinetic energy
Question 3 options:
mass and volume
volume and speed
speed and postion
mass and speed
Answers
Answer:
the 4th one
Explanation:
kinitc energy formula is1/2mv^
there is mass ,and velocity (speed)
I hope it help
Answer:
Explanation:
Speed and position
will magnesium and fluorine atoms most likely form an ionic bond or a covalent bond? 15px
Answers
Magnesium and fluorine atoms will most likely form an ionic bond.
Ionic bonds are formed between elements with a large difference in electronegativity, which is the measure of an atom's ability to attract electrons towards itself. Magnesium and fluorine have a difference in electronegativity of 2.13, which is large enough to form an ionic bond.
In ionic bonds, one atom loses electrons and becomes a positively charged ion (cation), while the other atom gains electrons and becomes a negatively charged ion (anion). In this case, magnesium will lose two electrons to become Mg2+ and fluorine will gain one electron to become F-. These two ions will then attract each other electrostatically to form magnesium fluoride (MgF2), which is an ionic compound.
On the other hand, covalent bonds are formed between elements with a small difference in electronegativity, where atoms share electrons to achieve a stable electron configuration. Magnesium and fluorine have a large electronegativity difference, so they are unlikely to share electrons and form a covalent bond. Therefore, magnesium and fluorine will most likely form an ionic bond.
Learn more about electronegativity here:
https://brainly.com/question/3393418
#SPJ11
what is chica's email
Answers
how are we (brainly users) supposed to know. unless its just me that doesn't know.
sorry that this isn't really much help. :/
how does the reaction rate change when the nitrogen monoxide concentration is doubled and chlorine concentration is halved? defined term
Answers
When the concentration of nitrogen monoxide is doubled and the concentration of chlorine is cut in half, the reaction is second order in nitrogen monoxide, NO.
Is NOx equivalent to NO2?
Nitric oxide (NO), an odorless, colorless gas, and nitrogen dioxide (NO2), a reddish-brown gas with an offensive odor, are the two gases that are typically referred to as "nitrogen oxides" (NOx). Nitrogen dioxide is created when nitric oxide combines with oxygen or ozone in the atmosphere.
N2O is a laughing gas, right?
A non-flammable, colorless, and odorless gas is nitrous oxide. Despite not being flammable, nitrous oxide will nonetheless facilitate combustion to the same degree as oxygen. It produces a mood of exhilaration, therefore the moniker "laughing gas." The least effective inhalational anesthetic is nitrous oxide.
To know more about nitrigen monoxide visit:
https://brainly.com/question/1328380
#SPJ4
Why are small quantities of chlorofluorocarbons so harmful to the ozone layer? a. The chlorofluorocarbons act like ultraviolet radiation causing large amount of ozone to be produced. b. The chlorine from the chlorofluorocarbons reacts with free molecules of oxygen causing a stop in ozone production. c. Free oxygen atoms can replace the chlorine in chlorine monoxide, releasing a free atom of chlorine which can then recombine with an oxygen atom in ozone, destroying more ozone. d. Chlorofluorocarbons absorb ultraviolet radiation, preventing the formation of ozone.
Answers
Answer:
Why are small quantities of chlorofluorocarbons so harmful to the ozone layer? Free oxygen atoms can replace the chlorine in chlorine monoxide, releasing a free atom of chlorine which can then recombine with an oxygen atom in ozone, destroying more ozone.
Explanation:
The statement for small quantities of chlorofluorocarbons so harmful to the ozone layer is "Free oxygen atoms can replace the chlorine in chlorine monoxide, releasing a free atom of chlorine which can then recombine with an oxygen atom in ozone, destroying more ozone."
What is ozone layer?The ozone layer is a thin layer of air in the Earth's atmosphere that absorbs nearly all of the sun's damaging UV radiation.
What is CFCs?CFCs (chlorofluorocarbons) are harmless and nonflammable compounds made up of carbon, chlorine, and fluorine atoms.
The earth's protective ozone layer is destroyed by chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and halons, which shield the earth from damaging ultraviolet (UV-B) rays released by the sun. CFCs and HCFCs also warm the earth's lower atmosphere, causing global climate change.
When some substances are exposed to high UV radiation in the stratosphere, they emit chlorine or bromine. Ozone-depleting chemicals are compounds that contribute to ozone depletion (ODS). Chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), carbon tetrachloride, and methyl chloroform are examples of ODS that produce chlorine. Halons and methyl bromide are two ODS that emit bromine.
Because there isn't much ozone in the atmosphere, what little there is is critical for protecting the Earth's surface from excessive UV light from the Sun. It turns out that it reacts with chlorine, which means that chlorine effectively eliminates ozone.
When the chlorine in CFCs combines with ultraviolet light, it releases chlorine, which then reacts with ozone, reducing the protection humans get from ultraviolet light, allowing more CFCs to release chlorine, and so on. Multiple ozone molecules will interact with one free chlorine atom, which is free because UV light has hit the CFC molecule. As a result, the damage it can cause is likely to be significantly more than you might imagine.
Hence the correct option is c.
Learn more about ozone layer and CFCs here
https://brainly.com/question/14330630
#SPJ2