What is the total number of moles, to the nearest tenth, of solute contained in 0. 50 liter of 3. 0 M HCl?
Answers
The total number of moles of solute (HCl) in 0.50 L of 3.0 M HCl is 1.5 moles.
To determine the total number of moles of solute in a solution, we can use the formula:
moles of solute = concentration of solution x volume of solution
In this case, we are given that the volume of the solution is 0.50 L and the concentration of the solution is 3.0 M HCl.
Using the formula above, we can calculate the number of moles of HCl in the solution:
moles of HCl = 3.0 M x 0.50 L
moles of HCl = 1.5 moles
This result can be explained by the fact that the concentration of a solution is defined as the amount of solute (in moles) per unit volume of the solution (in liters).
to know more about moles refer here:
https://brainly.com/question/31597231#
#SPJ11
Related Questions
GIVING BRAINLYYY
Use the general trends of the periodic table to answer the following question.
Platinum (Pt) and gold (Au) are sometimes called noble metals because they are less reactive than other metals. Based on this evidence and the periodic table, which other element is likely to be less reactive?

Answers
According to the electronic configuration, along with platinum and gold silver is considered to be a noble metal.
What is electronic configuration?
Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/29757010
#SPJ2
the vast majority of the geologic record contains well-preserved fossils that we can use to develop relative timing relationships among rock units.
Answers
This helps us to establish the relative ages of the rock formations based on the order of their deposition. The use of fossils in this manner is known as biostratigraphy, and it is an important tool in the field of geology.
The statement "the vast majority of the geologic record contains well-preserved fossils that we can use to develop relative timing relationships among rock units" is true. The presence of well-preserved fossils in the rock units is the basis for the relative timing relationships. These relationships can be used to establish the relative ages of rock formations by determining which layers were deposited first, second, third, and so on.In many cases, the fossils themselves can be used to establish the relative ages of the rock formations. This is because different types of fossils are associated with different geological time periods. For example, the fossils of trilobites are commonly found in rocks that are millions of years old, while the fossils of dinosaurs are typically found in much younger rocks.In conclusion, the vast majority of the geologic record contains well-preserved fossils that we can use to develop relative timing relationships among rock units. This helps us to establish the relative ages of the rock formations based on the order of their deposition. The use of fossils in this manner is known as biostratigraphy, and it is an important tool in the field of geology.
To know more about geology visit:
https://brainly.com/question/1047182
#SPJ11
Qué tipo de energía tiene un sacapuntas
Answers
Answer:
creo q es electrico
Explanation:
A hypothetical element has an atomic weight of 42.8452 amu. There are three main isotopes of this element with mass numbers of 41, 43 and 44. The 44 amu isotope has a 8% abundance. What is the percent abundance of the isotope with the mass number of 41?
1. 7.740%
2. 12.04%
3. 92.26%
4. 46.00%
5. 12.34\%
Answers
Answer:
Answer is 46.00%
Explanation:
absolute mass= A1F1 +A2F2
100
42.8452= 44×8% + 41×( 100--F2)
100
so you get
F2= 46.00%
am not sure
PLEASE HELP DUE TOMORROW!!!!
Percent Yield WS
4 did not fit cause it was on the back
4. Ammonium nitrate will decompose explosively at high temperatures to form nitrogen, oxygen, and water vapor.
2NH4NO3 (s) -> 2 N2 (g) + 4 H2O (g) + O2 (g)
What is the total number of liters of gas formed when 228 g NH4NO3 is decomposed? (Assume STP)

Answers
the total volume of gas formed when 228 g NH4NO3 is decomposed at STP is 35.9 L.
How do we calculate?Molar mass of NH4NO3 = 80 g/mol (14+4x4+3x16)
Number of moles of NH4NO3 = mass/molar mass = 228 g / 80 g/mol = 2.85 mol
According to the balanced chemical equation, 2 moles of NH4NO3 produce 1 mole of O2, 4 moles of H2O, and 2 moles of N2.
So, 2.85 mol of NH4NO3 will produce:
1/2 x 2.85 = 1.425 mol of O2
4 x 2.85 = 11.4 mol of H2O
2 x 2.85 = 5.7 mol of N2
Now we can use the ideal gas law to calculate the total volume of gas produced:
PV = nRT
At STP,
P = 1 atm
V = nRT/P = (1.425 + 11.4 + 5.7) mol x 0.08206 L atm K^-1 mol^-1 x 273.15 K / 1 atm = 35.9 L
Learn more about Molar mass at: https://brainly.com/question/21334167
#SPJ1
Write the molecular and empirical formulas of the following compound:
O-H
|
O-P-0-H
O-H
Answers
Answer:
the answer base on my test is H3O4P1
What is the symbol and number of protons in Arsenci
Answers
Answer:
As 33
Explanation:
Symbol: As
Atomic Number/ Number of Protons: 33
What is the frequency of 14000 X ray photons having an energy of 5.27*10-5 J/Hz
Answers
The frequency of the X - ray is \(7.95 \times 10^{28} \ Hz\).
The given parameters:
Energy of the X-ray, E = 5.27 x 10⁻⁵ JThe frequency of the wave is calculated as follows;
\(E = hf\\\\f = \frac{E}{h} \\\\\)
where;
h is Planck's constant = 6.626 x 10⁻³⁴ J/HzSubstitute the given parameters;
\(f = \frac{E}{h} \\\\f = \frac{5.27 \times 10^{-5} }{6.626 \times 10^{-34}} \\\\f = 7.95 \times 10^{28} \ Hz\)
Thus, the frequency of the X - ray is \(7.95 \times 10^{28} \ Hz\).
Learn more about frequency of radiation here: https://brainly.com/question/13400312
list 3 substances that have atoms which are bonded.
Answers
Answer:
Carbon, Hydrogen, Oxygen, Nitrogen, and Sulfur.
(Those are all the ones I know)
Example of 3 substances that have bonded atoms are :
Water ( H₂0 )Calcium Oxide ( CaO )Table salt ( NaCl )Substances that have chemically bonded atoms are generally known as molecules, while a compound is a substance made up by the chemical bonding of different atoms from different elements. there are some readily available substance in nature that have bonded atoms like ; water and Table salt.
Hence we can conclude that three ( 3 ) examples of substances that have bonded atoms are ; Water, Calcium Oxide and Table salt.
Learn more : https://brainly.com/question/21700212

H2(g) + I2(g) ↔2HI(g) + heat. If more I2 is added, in what direction will the equilibrium shift? Group of answer choices
Answers
Answer:
Towards the products, or to the right
Explanation:
There are no provided answer choices, but the answer should be to the right.
By Le Chatelier's principle, which basically can be summarized as "if you mess with chemistry, chemistry messes back", if more reactants are added, the equilibrium will shift to the right towards the products in order to make more products and counteract the increase in I₂.
a steam engine only converts 20 percent of its input energy into useful work?
Answers
Answer:
A heat engine is a type of engine, (like the motor in a car) that produces ... Heat engines do just the opposite; they take the energy from being warm ... using a flame to heat water into steam, then using the steam to turn a turbine. ... is the percentage of energy input that the engine can convert to useful work.
Explanation:
hope this helps
and i hope its right
Making Measurements Consider the following thermometer, and use it to answer the question. calibration Thermometer 200 difference between 2markedvalues #ofspacesbetween marked values 30 20 10 What is the nearest value that we can estimate the volume of this thermometer to? A. 0.1°C B. 0.01 C C. 0.00 °C D. 1 °C

Answers
Answer:0.1C
Explanation:
Answer: It is 1
Explanation: Got it correct on Acellus hope it helps!
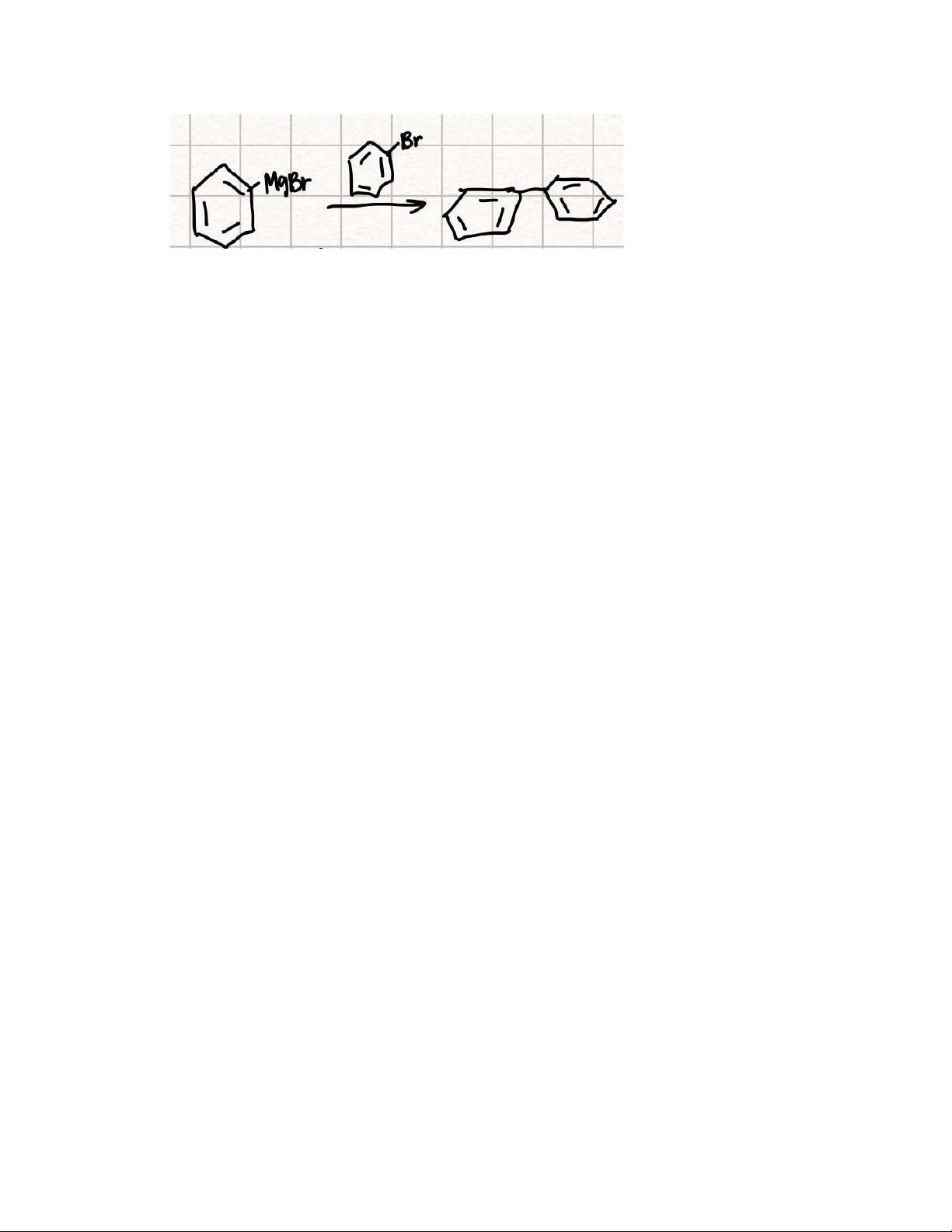
1. bromobenzene was added in increments during the grignard reaction to avoid unwanted side reactions. what was the unwanted side reaction? please draw the product of this unwanted side reaction. how was the unwanted side product removed during work up?
Answers
The unwanted side reaction if bromobenzene was added in increments during the Grignard reaction was a radical reaction that eventually led to the dimerization of two alkyl bromides.
The draw of the unwanted side reaction is shown in picture.
To remove the unwanted side during work up which is the biphenyl product, we can use petroleum ether.
About Grignard reaction:
Grignard reaction is the process addition of organomagnesium halide like alkyl/vinyl/aryl to any carbonyl group in aldehyde/ketone. The reaction is considered an important tool to form carbon-carbon bonds
Learn more about Grignard reaction here:
https://brainly.com/question/14133796
#SPJ4
Using the following equation, 2C2H6 +7O2 -->4CO2 +6H2O, if 2.5g C2H6 react with 170g of O2, how many grams of water will be produced?
Answers
The mass of water (H₂O) that would be produced is 4.5 g
StoichiometryFrom the question, we are to determine the mass of water that would be produced.
From the given balanced chemical equation
2C₂H₆ +7O₂ → 4CO₂ +6H₂O
This means
2 moles of C₂H₆ reacts with 7 moles of O₂ to produce 4 moles of CO₂ and 6 moles of H₂O
Now, we will determine the number of moles of each reactant present
For Ethane (C₂H₆)Mass = 2.5 g
Molar mass = 30.07 g
Using the formula,
\(Number\ of\ moles = \frac{Mass}{Molar\ mass}\)
Number of moles of C₂H₆ present = \(\frac{2.5}{30.07}\)
Number of moles of C₂H₆ present = 0.08314 mole
For Oxygen (O₂)Mass = 170g
Molar mass = 31.999 g/mol
Number of moles of O₂ present = \(\frac{170}{31.999}\)
Number of moles of O₂ present = 5.3127 moles
Since
2 moles of C₂H₆ reacts with 7 moles of O₂
Then,
0.08314 mole of C₂H₆ will react with \(\frac{7 \times 0.08314 }{2}\)
\(\frac{7 \times 0.08314 }{2}\) = 0.58198 mole
Therefore,
0.08314 mole of C₂H₆ reacts with 0.58198 mole of O₂ to produce 3 × 0.08314 moles of H₂O
3 × 0.08314 = 0.24942 mole
Thus, the number of moles of water (H₂O) produced is 0.24942 mole
Now, for the mass of water that would be produced,
Using the formula,
Mass = Number of moles × Molar mass
Molar mass of water = 18.015 g/mol
Then,
Mass of water that would be produced = 0.24942 × 18.015
Mass of water that would be produced = 4.4933 g
Mass of water that would be produced ≅ 4.5 g
Hence, the mass of water (H₂O) that would be produced is 4.5 g
Learn more on Stoichiometry here: https://brainly.com/question/14271082
plzzz help me i promise will mark u brainiest

Answers
Answer:
d) water
Explanation:
Answer:
C) sodium chloride
list essential conditions for pressure
Answers
The essential conditions for pressure are high temperature and minimum volume.
What is pressure?Pressure is defined as the force applied on an object perpendicular to it's surface per unit area over which it is distributed.Gauge pressure is a pressure which is related with the ambient pressure.
There are various units by which pressure is expressed most of which are derived units which are obtained from unit of force divided by unit of area . The SI unit of pressure is pascal .
It is a scalar quantity which is related to the vector area element with a normal force acting on it.It is distributed over solid boundaries and across arbitary sections of fluid normal to the boundaries at every point.
Learn more about pressure,here:
https://brainly.com/question/14760196
#SPJ9
at 4.00 l , an expandable vessel contains 0.864 mol of oxygen gas. how many liters of oxygen gas must be added at constant temperature and pressure if you need a total
Answers
First, we'll look at the ideal gas equation,
PV = nRT
The temperature and pressure are said to be constant; Additionally, R is a constant already. Along these lines, we get:
V = constant * n
The direct proportional equation is as follows: As a result, we get:
V/n = constant
V₁/n₁ = V₂/n₂
Replace V₂ with the qualities and address.
V₂ = (4 * 1.48) / 0.864
V₂ = 6.85
In the end, 6.85 Liters of gas must be present, so we must add:
6.85 - 4 = 2.85 liters
The volume of a gas is directly proportional to its mole volume at a fixed temperature and pressure.
To learn more about ideal gas here
https://brainly.com/question/28257995
#SPJ4
Q- At 4.00 L, an expandable vessel contains 0.864 mol of oxygen gas. How many liters of oxygen gas must be added at constant temperature and pressure if you need a total of 1.48mol of oxygen gas in the vessel?
the third law of thermodynamics describes the entropy of a: select the correct answer below: solid liquid gas all of the above
Answers
The third law of thermodynamics describes the entropy of a: solid.
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius). This law implies that at absolute zero, a perfectly ordered and pure crystalline solid will have zero entropy.
The third law of thermodynamics is not specific to liquids or gases but applies to solids. In a solid, the molecules are highly ordered and have fixed positions in a regular lattice structure. As the temperature decreases towards absolute zero, the thermal motion of the molecules reduces, and the system becomes more ordered, resulting in a decrease in entropy.
In contrast, liquids and gases have higher entropy compared to solids at absolute zero because their molecules have more freedom of movement and are not as tightly arranged. Therefore, the third law of thermodynamics specifically addresses the entropy of solids and does not apply to liquids or gases.
To learn more about law of thermodynamics, here
https://brainly.com/question/1368306
#SPJ4
A solution at 25°C is 1.0 x 10^-5 M H3O+. What is the concentration of OH- in this solution
Answers
Answer:
1.0 × 10–9 M OH–
Explanation:
pH = -Log[H+]
pOH = -Log[OH-]
But;
pH + pOH = 14
Therefore;
[H+] + [OH-] = 1.0 × 10^-14 M
Therefore;
[OH-] = 1.0 × 10^-14 M - (1.0 × 10^–5 M)
= 1.0 × 10^-9 M OH–
Explain why butter and graphite have different melting points?
Answers
Answer:
Differences in melting points between graphite and butter is how their atoms are fixed structurally. Graphite consists of carbon atoms that have covalent bonding with a compact hexagonal arrangement that gives a lot of stability to the compound and makes its melting point elevated to approximately 3500oC.
The allotropes of carbon are diamond and graphite. The allotropes can have same or different physical or chemical properties. Therefore, differences in melting points between graphite and butter is due difference in structure.
What are allotropy?Allotropy is a property by which an element exist in more than one form. The different forms of element are called allotropes.
The features of graphite are :
1.Graphite form strong covalent bonds.
2. Graphite has delocalized electrons but they don't have metallic bonding.
3. There are total four electrons per carbon but only three electrons are available for covalent bond. One electron per carbon is responsible for delocalization.
4. Graphite forms a giant structures.
Graphite consists of carbon atoms that have covalent bonding with a compact hexagonal arrangement that gives a lot of stability to the compound and makes its melting point elevated.
Therefore, differences in melting points between graphite and butter is due difference in structure.
To know more about allotropy, here:
https://brainly.com/question/16914774
#SPJ2
What is the relation for entropy change for reversible process?
Answers
If the process is irreversible, the entropy change may be positive, negative, or zero, depending on the direction of heat flow.
The relation for entropy change for a reversible process is given by the equation ΔS = Qrev/T, where ΔS is the change in entropy, Qrev is the heat absorbed or released during the reversible process, and T is the temperature at which the process occurs. In a reversible process, the entropy change is positive for an increase in temperature and negative for a decrease in temperature. This equation is important in thermodynamics because it allows us to calculate the change in entropy for a reversible process and determine the maximum efficiency of a heat engine.
To learn more about entropy click here https://brainly.com/question/13999732
#SPJ11
The density of liquid ethanol is 0. 789 g/ml. What is its density in units of lb/in 3? (2. 54 cm = 1 in. , 2. 205 lb = 1 kg).
Answers
Since liquid ethanol has a density of 0.789 g/mL, this means that 0.789 g/mL times 0.0624 lb/g equals 0.0489 lb/mL or that 0.0489 lb/mL times 61.0237 in3/mL is 2.984 lb/in3.
Two conversions must be made: from g/mL to lb/mL and from lb/mL to lb/in3 for the density of liquid ethanol to be changed from g/mL to lb/in3.
First, we convert g/mL to lb/mL using the formula: 0.0489 lb/mL = 0.789 g/mL x 0.00220462 lb/g.
Following that, we translate lb/mL to lb/in3 as follows: 0.0489 lb/mL x 61.0237 in3/mL = 2.984 lb/in3.
As a result, liquid ethanol has a density of 2.984 pounds per cubic inch. Through the use of conversion factors, the density of liquid ethanol, which is 0.789 g/mL, may be changed to 2.984 lb/in3.
learn more about liquid ethanol here:
https://brainly.com/question/15111978
#SPJ4
Why are perchlorate salts unusually hazardous?
- They are toxic and volatile.
- Some are shock-sensitive.
- They are strong bases.
- They are water-reactive.
Answers
Perchlorate salts are unusually hazardous primarily because they are toxic and volatile.
Perchlorate salts are unusually hazardous primarily because they are toxic and some are shock-sensitive. Their toxicity can pose a risk to human health and the environment, while their shock-sensitive nature can cause them to react violently upon impact, potentially leading to accidents or explosions. Perchlorate salts are unusually hazardous due to several reasons. Firstly, they are toxic and volatile, meaning they can easily vaporize and become airborne, increasing the risk of inhalation and absorption through the skin. Secondly, some perchlorate salts are shock-sensitive, meaning they can easily detonate or explode when subjected to impact or friction.
Additionally, perchlorate salts are strong bases, which can cause severe chemical burns and damage to tissues and organs upon contact. Finally, they are also water-reactive, which can cause them to release oxygen and hydrogen gas, leading to potential fire and explosion hazards. Overall, the unique combination of these characteristics makes perchlorate salts particularly hazardous and requires careful handling and disposal.
Learn more about Perchlorate salts here: brainly.com/question/8269990
#SPJ11
What is the organic compound?

Answers
Answer:
propa-1-nol
Explanation:
easy clapp
Which of the following is a
possible way to describe the HCI
component in the reaction below?
2HCl(aq) + Ca(OH)₂(aq)
2H₂O(1) +CaCl₂(aq)
A. 2 atoms HCI
B. 2LHCI
C. 36.45 g HCI
D. 2 moles HCI
Answers
2 moles The HCI component of the process 2HCl(aq) + Ca(OH)2(aq) 2H2O(1) + CaCl2 could be described as HCI.(aq).
What exactly does the word "performance" mean in the context of HCI?In the context of HCI, the meaning of "performance" is also crucial. In this context, "performance" refers to both the effectiveness with which a task is carried out and the calibre of the output that the task produces.
Of the following, which one is an HCI principle?Perception, behaviour models, descriptive modeling, and those covered by Schneiderman's 8 rules are the four basic principles of HCI. The user interface needs to be designed in a manner that anyone can use it without any help.
To know more about moles visit:-
https://brainly.com/question/26416088
#SPJ1
Where is the current North magnetic Pole?
Answers
Answer:
The current location of the North Magnetic Pole is in the Arctic Ocean, near the coast of northern Canada, specifically in the region of northern Quebec and Ellesmere Island in the Canadian Arctic Archipelago.
Explanation:
However, it's important to note that the North Magnetic Pole is not a fixed point on the Earth's surface, but it is instead constantly moving. The North Magnetic Pole is moving away from Canada and towards Siberia at a rate of about 55 km (34 miles) per year. This movement is caused by changes in the Earth's magnetic field and is known as the "wandering" of the magnetic poles. The exact location of the North Magnetic Pole can be determined by regular measurements and it is used as a reference point for navigation.
80 calories must be lost to make water change into ice at 0° C. True False
Answers
* GIVING BRAINLIEST*
3Ca+2 FeCl3 -> 3CaCl2 + 2Fe
Calcium metal + Iron Chloride -> Calcium Chloride + Iron metal
Identify the reason that atoms react with each other.
Answers
i think its double replacement if i'm not mistaken
A scientist finds a fossil that she thinks might make a good index fossil.
Which characteristic does this fossil most likely have?
Answers
Answer: A fossil will usually have remains or impressions of an animal or plant.
Answer:
the answer is b
Explanation:
trust me
4NH3(g)+6NO(g) yields 5N2(g)+6H2O(l) How many moles NO are required to completely react with 2. 45 mol NH3
Answers
As per the balanced reaction given, 4 moles of ammonia reacts with 6 moles of NO. Therefore, the number of moles of NO required to react with 2.45 moles of ammonia is 3.46.
What is nitric oxide?Nitric oxide is a covalent compound formed from the electron sharing between nitrogen and oxygen atom. Nitric oxide is a common gas found in atmosphere.
NO reacts with ammonia gas produces nitrogen gas and water as per the balanced chemical equation given. It is clear that 4 moles of ammonia reacts with 6 moles of NO.
The number of moles of NO required to react with 2.45 moles of ammonia is calculated as follows:
number of moles of NO = 2.45 × 6 /4 = 3.46.
Therefore, 3.46 moles of NO is required to completely react with 2.45 moles of ammonia.
To find more on nitric oxide, refer here:
https://brainly.com/question/29357729
#SPJ4