Answers
Answer:
Work is a force causing the movement or displacement of an object
law of conservation mass:
1. Atoms cannot be created or destroyed in a chemical reaction.
2. Molecules cannot be created or destroyed in a chemical reaction.
3. Compounds cannot be created or destroyed in a chemical reaction.
4. Heat cannot be created or destroyed in a chemical reaction.
Related Questions
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
Which alignment of the Sun, Moon, and Earth causes a lunar eclipse? А. B. C. D.

Answers
Answer:
A.
Explanation:
I'm awesome
The Universal Space Agency wants to know the results of your Sim investigation. Write your report to the lead chemist, explaining why drops of liquid water appeared on the outside of the cold, but not the warm, soda can. Use evidence from your investigation in your explanation.
Answers
Answer:
due to warm temperature
Explanation:
Explanation:
There is water present in liquid form instead of liquid nitrogen on the outer side of the can because of the temperature present in the surrounding environment. The temperature around the can is little warm which is favourable for the water but not for the liquid nitrogen. The liquid nitrogen present in liquid state when the temperature of the surrounding is too cold. if it is warm, the liquid nitrogen starts boiling and converts into gaseous state so that's why the warmer temperature is responsible for the presence of water not the liquid nitrogen.
Mylanta a common antacid contains magnesium hydroxide Mg (OH)2.How many miles of magnesium hydroxide are in 75.0g of magnesium hydroxide?
Answers
To answer this question, we need to convert grams to moles and then use the Avogadro's number to convert moles to molecules.
The molar mass of Mg(OH)2 is 58.32 g/mol.
First, we need to find the number of moles of Mg(OH)2 in 75.0 g.
75.0 g / 58.32 g/mol = 1.287 mol
Next, we need to convert moles to molecules.
1.287 mol x 6.022 x 10^23 molecules/mol = 7.75 x 10^23 molecules
Finally, we can use the molecular formula of Mg(OH)2 to calculate the number of miles of Mg(OH)2.
1 molecule of Mg(OH)2 contains 3 atoms of oxygen (O) and 2 atoms of hydrogen (H).
So, the total number of miles of Mg(OH)2 is:
7.75 x 10^23 molecules x 2 miles/molecule = 1.55 x 10^24 miles
Therefore, there are 1.55 x 10^24 miles of magnesium hydroxide in 75.0 g of magnesium hydroxide.
What has 2 protons and 2 neutrons in the center of the atom
Answers
Answer:
HELIUM
Explanation:
HELIUM HAS 2 PROTON I
49.The 1H NMR spectrum of a compound with formula C7H14O gives a doublet at 9.2 ppm. Which of these structures is a possible one for this compound?
Answers
Answer: The possible structure for this compound is 2,4-dimethyl-3-pentanone
Explanation:
It should be noted that, If the 1H NMR spectrum of a compound with formula C7H14O gives a doublet at 9.2 ppm. The possible structure for this compound is 2,4-dimethyl-3-pentanone
What is the best method of separating the mixture of sand and fine salt?
Answers
By using filtration, the sand and fine salt can be effectively separated based on their difference in particle size, providing a clean separation of the two components.
Filtration is a separation technique that takes advantage of the difference in particle size between sand and salt. It involves passing the mixture through a porous material, such as filter paper or a filter funnel, which allows the liquid (saltwater) and small salt particles to pass through while retaining the larger sand particles.
Here's how the filtration process can be carried out:
1. Set up a filter apparatus with a funnel and filter paper or a filter flask.
2. Place the mixture of sand and salt in a beaker or a flask.
3. Slowly pour the mixture into the filter paper or funnel, allowing the liquid (saltwater) to pass through while retaining the sand on the filter paper.
4. Once the liquid has passed through completely, the sand will be left behind on the filter paper or in the filter flask.
5. Carefully remove the sand from the filter paper or filter flask, and the saltwater solution can be collected separately.
For more such questions on filtration
https://brainly.com/question/29756050
#SPJ8
Predict the decay process and write the nuclear equation of Cf- 255
Answers
Answer:
curium
−
243
,
252
/
99
Es,
251
/
98
Cf,
214
/
82
Pb
Explanation: Im not very good with this but here ya go!
Can someone please help me with this question. I got half of the question and I am stuck on the rest.

Answers
The mean of the data set is approximately 4.0626, and the 90% confidence interval is [4.060925, 4.064275].
What is the mean and 90% confidence interval of the given data?The sample mean (x) is calculated as follows:
x = (4.0620 + 4.0550 + 4.0650 + 4.0740 + 4.0550 + 4.0660) / 6
x ≈ 4.0626 (rounded to four decimal places)
The 90% confidence interval is calculated as follows;
Standard deviation (s):
(4.0620 - 4.0626)² = 0.00000036
(4.0550 - 4.0626)² = 0.00000576
(4.0650 - 4.0626)² = 0.00000006
(4.0740 - 4.0626)² = 0.00001328
(4.0550 - 4.0626)² = 0.00000576
(4.0660 - 4.0626)² = 0.00000012
average of the squared differences:
(0.00000036 + 0.00000576 + 0.00000006 + 0.00001328 + 0.00000576 + 0.00000012) / 6 ≈ 0.00000624
s = √(0.00000624)
s ≈ 0.002496
the standard error of the mean (SEM):
SEM = 0.002496 / √6
SEM ≈ 0.001018
For a 90% confidence interval, the z value is approximately 1.645.
ME = 1.645 * 0.001018 ≈ 0.001675
CI = x ± ME
CI = 4.0626 ± 0.001675
CI ≈ [4.060925, 4.064275]
Learn more about mean and confidence intervals at: https://brainly.com/question/20309162
#SPJ1
Compare and contrast diffusion and convection and the impact on dispersal of air pollution.
Answers
Diffusion and convection are two distinct processes that play a role in the dispersal of air pollution, but they differ in how they transport pollutants and their impact on dispersion.
Diffusion refers to the spontaneous movement of particles from an area of higher concentration to an area of lower concentration. It occurs due to random thermal motion of molecules. In the context of air pollution, diffusion allows pollutants to spread out gradually, dispersing them in various directions. However, diffusion alone is a relatively slow process, particularly for large-scale dispersion, and it may not be effective in rapidly distributing pollutants over long distances.
Convection, on the other hand, involves the transfer of heat energy through the movement of a fluid, such as air or water. In the atmosphere, convection occurs as warm air rises, creating upward currents and transporting pollutants vertically. As the air rises, it carries pollutants to higher altitudes, which can lead to their dispersion over larger areas. Convection is a more efficient process for the vertical transport and dispersion of pollutants compared to diffusion.
The impact of diffusion and convection on the dispersal of air pollution can vary. Diffusion primarily affects local dispersion, allowing pollutants to spread out in the immediate vicinity of emission sources. It is more significant in areas with minimal air movement. Convection, on the other hand, can facilitate the long-range transport of pollutants, particularly when large-scale weather systems are involved. Convection can carry pollutants over greater distances and contribute to regional or even global dispersion, depending on weather patterns.
In summary, diffusion and convection are both involved in the dispersal of air pollution, but they differ in the mechanisms of transport and the scale of dispersion. Diffusion leads to gradual spreading of pollutants locally, while convection enables vertical transport and dispersion over larger areas, including long-range transport depending on weather conditions. Understanding the interplay between these processes is crucial for assessing the extent and impact of air pollution.
For more question on Diffusion
https://brainly.com/question/94094
#SPJ8
What is the difference between the structure of simple and complex carbohydrates?
Answers
Answer:
Simple carbs break down easier and contain sugars. Complex carbs contain starch and fiber.
Explanation:
Answer Image result for What is the difference between the structure of simple and complex carbohydrates?
Simple vs Complex Carbs. Carbohydrates are sugars that come in 2 main forms – simple and complex. This is also referred to as simple sugars and starches. The difference between a simple and complex carb is in how quickly it is digested and absorbed – as well as it's chemical structure.
You are taking a scuba diving training course. The instructor is discussing
your scuba tanks, and tells you they have a volume of about 11 L and hold
enough gas for a one-hour dive. The instructor also tells you that you take in
about 2 L of gas with each breath. Which is the best explanation for why 11 L
of gas lets you breathe for one hour?
A Because of the high pressure of the water, the amount of gas
needed for each breath decreases.
B It is very cold underwater. This decreases the pressure inside the
tank and allows exhaled gases to be stored and breathed in
again.
© The gas is under pressure in the tank. As pressure increases,
volume decreases. A small tank can therefore hold a large
amount of gas.
Underwater the gas becomes cold enough for it to become a
solid. The solid vaporizes a little at a time to allow you to breathe
for longer
Answers
Answer:
C
Explanation:
The deeper you go into water, the greater pressure there is on everything. This compresses the air inside and causes the volume to shrink.
Sometimes air is measured in bars. Let's assume the Earth's surface pressure is 1. Let's also say that your lungs have 5.5L and the tank has 11L. That's only 2 breathes, but under 200 bars of pressure deep in the ocean? That's 200 x 11, which equals 2200L. That's about 400 breathes.
In addition, if making liters out of thin air doesn't make any sense, there's an alternative where you divide your liters per breath by the bar pressure. So instead of 200 x 11, you can take your 5.5 from your lungs and divide it by 200. That small number, in this case, 0.0275, can serve as how much air you breath deep in the ocean.
. What is the relationship between frequency and energy of light?DirectIndirectExponentialLogarithmic
Answers
In an electromagnetic spectrum, we can see a wide variety of possible electromagnetic radiations, which will differ in wavelength, energy, frequency and some other characteristics. The relationship of wavelength and energy is inversely proportional, which means, long wavelengths is equal to low frequency and energy. Energy of the radiation and frequency are directly proportional, which means that if we have a radiation with a high energy, we will also have a high frequency. Therefore, the answer will be the 1st option, Direct
When an alarm sounds in the laboratory, it is important to respond _______ and remain ______. Listen for any other instructions, turn off any _______ in use, and be prepared to leave immediately.
1) calmly
2) quiet
3) equipment
Answers
When an alarm sounds in the laboratory, it is important to respond quickly and remain calm. Listen for any other instructions, turn off any equipment in use, and be prepared to leave immediately.
What is laboratory?
Laboratory is a place where scientific experiments and measurements are performed. It is equipped with various types of equipment and instruments to carry out experiments and tests. Laboratories are usually used by scientists and technicians to conduct experiments, analyze samples, and conduct research. It is an area that is designed to be kept clean and organized in order to ensure safety and accuracy when conducting experiments. Laboratories can be found in many different settings such as hospitals, universities, research and development centers, and manufacturing facilities.
To learn more about laboratory
https://brainly.com/question/29482908
#SPJ4
For the reaction
4PH3(g)↽−−⇀6H2(g)+P4(g)
the equilibrium concentrations were found to be [PH3]=0.250 M, [H2]=0.580 M, and [P4]=0.750 M.
What is the equilibrium constant for this reaction?
c=
Answers
The equilibrium constant (Kc) for the given reaction is approximately 16.448. The value of Kc indicates the relative concentrations of reactants and products at equilibrium. In this case, a Kc greater than 1 suggests that the products (H2 and P4) are favored at equilibrium, indicating that the forward reaction is more favorable.
To determine the equilibrium constant (Kc) for the given reaction:
4PH3(g) ↔ 6H2(g) + P4(g)
We can write the equilibrium constant expression based on the stoichiometric coefficients:
Kc = ([H2]^6 * [P4]) / ([PH3]^4)
Substituting the given equilibrium concentrations:
[PH3] = 0.250 M
[H2] = 0.580 M
[P4] = 0.750 M
We can plug in these values into the equilibrium constant expression:
Kc = ([0.580]^6 * [0.750]) / ([0.250]^4)
Kc = (0.0860128 * 0.750) / (0.00390625)
Kc = 16.448
for more question on equilibrium
https://brainly.com/question/18849238
#SPJ8
Why is k+ much more stable than k2+?
Answers
How many grams of C are required to react with 64.6 g of Fe2O3 ?
Answers
Answer:
14.6 g
Explanation:
Fe₂O₃ + 3 C → 2 Fe + 3 CO₃
First, convert grams of Fe₂O₃ to moles. The molar mass is 159.69 g/mol.
(64.6 g)/(159.69 g/mol) = 0.4045 mol
Now, use stoichiometry to convert moles of Fe₂O₃ to moles of C. By looking at the chemical equation, you can see that for every 1 mole of Fe₂O₃, you need 3 moles of C. Use this relationship.
(0.4045 mol Fe₂O₃) × (3 mol C/1 mol Fe₂O₃) = 1.214 mol C
Now, convert moles of C to grams. The molar mass is 12.01 g/mol.
(1.214 mol C) × (12.01 g/mol) = 14.58 g
You need to use significant figures.
14.58 g ≈ 14.6 g
Calculate ph of 1,0 mol/l of solution NH4Cl
Answers
Answer:
4.74.
Explanation:
The equilibrium equation for the hydrolysis of NH4+ is:
NH4+ + H2O ⇌ NH4OH + H+
The Cl- ion is a strong base and will not undergo hydrolysis in water. Therefore, the Cl- ion will not affect the pH of the solution.
Use the equilibrium constant for the hydrolysis of NH4+ (Kb) to calculate the pH of the solution.
The expression for the equilibrium constant for the hydrolysis of NH4+ is:
Kb = [NH4OH][H+] / [NH4+]
Given that the concentration of NH4Cl is 1.0 mol/L, the concentration of NH4+ ions is also 1.0 mol/L.
The concentration of hydroxide ions (OH-) can be calculated from the Kb expression:
Kb = [NH4OH][H+] / [NH4+]
[OH-] = Kb * [NH4+]
Knowing that the Kb for NH4+ = 1.810^-5
[OH-] = 1.810^-5 * 1.0 = 1.8*10^-5 M
The pH of the solution is the negative logarithm of the hydroxide ion concentration:
pH = -log([OH-])
pH = -log(1.8*10^-5)
pH = 4.74
Therefore, the pH of 1.0 mol/L solution of NH4Cl is 4.74.
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
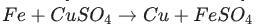
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
32.A student set up the following investigation. An artificial membrane separates two fluid-filledcompartments. This membrane is permeable to water but impermeable to sucrose. Bothcompartments initially hold the same amount of fluid, which consists of sucrose dissolved inwater. The fluid in compartment A is a 20% sucrose solution. The fluid in compartment B is a35% sucrose solution.WILLSelectively permeable membraneCompartment ASucrosemoleculesCompartment BWhich prediction is correct?A. Water will move from compartment A to compartment B by osmosis.B. Water will move from compartment B to compartment A by osmosis.C. Sucrose will move from compartment A to compartment B by diffusion.D. Sucrose will move from compartment B to compartment A by diffusion.ChaErKe
Answers
The movement of water would be from the compartment A to the compartment B.
What is Osmosis?We know that when we talk about the process of osmosis we are talking about how water moves from the region of lower concentration to the medium of the higher concentration.
In this case, we know that compartment A is a 20% sucrose solution while compartment B is a 35% sucrose solution. The partitions that separates the solutions is a semi permeable membrane thus we expect that osmosis would occur.
Learn more about Osmosis:https://brainly.com/question/1799974
#SPJ1
Why are watersheds important for the maintenance of water quality? Choose all that apply.
Select 2 correct answer(s)
Question 3 options:
they can transfer pollutants
their soil and plants can filter and clean water
they cause precipitation and acid rain
they cause floods
Answers
Answer:
its A and D
Explanation:
i took the quiz
Answer:
a and d
Explanation:
Which of the following elements has six valence electrons?
Be
B
C
N
O
Answers
A stock solution is 2.5 M K2Cr2O7.How many cm3 of this solution you need to dilute to make 50cm3 of 0.05M K2Cr2O7
Answers
The volume needed to dilute a stock solution to make 50cm3 of 0.05M K2Cr2O7 is 1cm³.
How to calculate volume?The volume needed to dilute a stock solution can be calculated using the following formula:
C1V1 = C2V2
Where;
C1 = initial concentrationC2 = final concentrationV1 = initial volumeV2 = final volumeAccording to this question, a stock solution is 2.5 M K2Cr2O7 and needs to be diluted to make 50cm3 of 0.05M K2Cr2O7. The volume needed can be calculated as follows:
2.5 × V1 = 50 × 0.05
2.5V1 = 2.5
V1 = 1cm³
Therefore, the volume needed to dilute a stock solution to make 50cm3 of 0.05M K2Cr2O7 is 1cm³.
Learn more about volume at: https://brainly.com/question/20458032
#SPJ1
What volume of a 4.4 M solution of HCI contains 303 g of HCI if the molar mass of HCI is 36.46 g/mol?
O 1.88 L
O 1.23 L
O2.13 L
O 2.45 L
Answers
Answer:
A.) 1.88 L
Explanation:
To find the volume of HCl , you need to (1) convert grams to moles (using the molar mass) and then (2) calculate the volume (using the molarity ratio).
(Step 1)
Molar Mass (HCl): 36.46 g/mol
303 grams HCl 1 mole
------------------------- x ---------------------- = 8.31 moles HCl
36.46 grams
(Step 2)
Molarity = 4.4 M
Moles = 8.31 moles
Molarity = moles / volume (L) <----- Molarity ratio
4.4 M = 8.31 moles / volume <----- Insert values
(4.4 M) x volume = 8.31 moles <----- Multiply both sides by volume
volume = 1.88 <----- Divide both sides by 4.4
A student is researching how chemical reactions occur and how temperature impacts the rate of the reaction. She
measures how long it takes for 5 grams of calcite to dissolve in a strong solution of hydrochloric acid at different
temperatures. Her data is shown in the graph

Answers
Based on the data shown in the graph, the rate of reaction is directly proportional to the temperature of a reaction.
What is the rate of a reaction?The speed at which a chemical reaction occurs is called the reaction rate or rate of reaction. The rate of a reaction is proportional to the increase in product concentration per unit time and the decrease in reactant concentration per unit time.
The rate of a reaction is affected by the following:
the temperature of the reaction - the rate of reaction is directly proportional to the temperature of a reaction. Hence, the rate of a reaction increases with an increase in temperature.
presence of a catalyst - the rate of a reaction increases with the addition of a catalyst. A catalyst speeds up the rate of a reaction.
the surface area of the reactants - the rate of a reaction increases with an increase in the surface area of the reactants,
Learn more about the rate of reaction at: https://brainly.com/question/25724512
#SPJ1
Answer:
At higher temperatures, chemical reactions occur more quickly.
Explanation:
edmentum
What causes an electron in an atom to move to a higher energy level?
A. It emits a specific amount of light energy.
B. It absorbs chemical energy.
C. It absorbs a specific amount of light energy.
D. It releases chemical energy
Answers
What causes an electron in an atom to move to a higher energy level it that it absorbs a specific amount of light energy. C
Excited state of an atomAn atom is in its excited state when it moves from a lower energy level to a higher energy level by absorbing a specific amount of light energy.
If an atom is in a lower energy state, it is said to be in its ground state.
Thus, what causes an electron in an atom to move to a higher energy level it that it absorbs a specific amount of light energy
Learn more about excited state here:
https://brainly.com/question/2066549
#SPJ1
The bright-line spectra of four elements, G,J, L, and M, and a mixture of at
least two of these elements are given below.
Which elements are present in the mixture?
M
Mixture
750
750
G and J
G and L
M, J, and G
M, J, and L
700
700
650
650
Bright-Line Spectra
600
600
550 500
550
Wavelength (nm)
500
450
450
400
400
.
Answers
Based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
From the given bright-line spectra and the spectrum of the mixture, we can determine the elements present in the mixture by comparing the specific wavelengths observed. Examining the bright-line spectra, we can identify that G has a distinct wavelength at 650 nm, J at 600 nm, L at 550 nm, and M at 500 nm.
Looking at the spectrum of the mixture, we can observe two prominent wavelengths, 650 nm and 600 nm. These correspond to the wavelengths of G and J, respectively. Since the spectrum of the mixture does not exhibit the wavelengths specific to L (550 nm) or M (500 nm), we can conclude that only G and J are present in the mixture.
Therefore, based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
This analysis relies on the principle that each element has characteristic wavelengths at which they emit light. By comparing the observed wavelengths in the mixture's spectrum with those of the individual elements, we can determine the elements present in the mixture.
Know more about wavelengths here:
https://brainly.com/question/10750459
#SPJ8
Predict the carbenoid addition products of the following reactions: trans-hex-3-ene + CH2I2, Zn(Cu) cis-hept-2-ene + CH2I2, Zn(Cu)
Answers
The reactions that result in the carbenoid addition products. Trans-hex-3-ene with CH2I2, Zn (a) (Cu). 3-Nitro-pent-2-ene. ALKENE HYBRIDIZATION When three atoms are joined to a carbon (that is, one of the bonds is a double bond).
What is the carbenoid addition products?
A gateway functional group is the double bond in an alkene. The basis for the remainder of your study of organic chemistry is laid by alkene reactions, which generate numerous additional functional groups. The most frequent reactions of double bonds change the pi bond into a sigma bond because single bonds (sigma bonds) are more stable than pi bonds. As an illustration, catalytic hydrogenation splits the H-H sigma bond into two C-H sigma bonds (Section 7-8). Exothermic (H° = approximately -80 to -120 kJ>mol or approximately -20 to -30 kcal>mol).
To learn more about carbenoid addition from given link
brainly.com/question/17094562
#SPJ4
How much total energy is released to cool 28.3 g of steam (water vapor) at 100.0°C to liquid water at 25.5°C? Water has a heat of vaporization of ± 40.7 kJ/mol and a specific heat of 4.184 J/g°C.
Answers
Answer:
\(\Delta H=-72870J=-72.9kJ\)
Explanation:
Hello,
In this case, for the computation of the total energy, we must consider two processes:
1. Condensation of steam (heat of condensation is the negative of heat of vaporization).
2. Cooling of hot water (we use the specific heat of water).
Thus, we write:
\(\Delta H=\Delta H_{cond}+\Delta H_{cooling}\)
For each term, we have:
\(\Delta H_{cond}=28.3g*\frac{1mol}{18g} *-40.7\frac{kJ}{mol} *\frac{1000J}{1kJ}= -63989.44J\)
\(\Delta H_{cooling}=28.3g*4.184\frac{J}{g\°C}*(25.5-100.0)\°C =-8880.54J\)
Therefore, the total energy results:
\(\Delta H=-63989.44J-8880.54J\\\\\Delta H=-72870J=-72.9kJ\)
Regards.
How many mL of 2.25M H2SO4 are needed to react completely with 69.9g BaO2

Answers
Answer:
4 millllllermeeters jb