What neutralizer is typically used with thio relaxers?.
Answers
The neutralizer that is typically used with thio relaxers is hydrogen peroxide (H2O2).
Thio relaxers are used to chemically straighten hair, and they work by breaking down the disulfide bonds in the hair. After the hair has been treated with the thio relaxer, a neutralizer is used to stop the chemical process and rebuild the disulfide bonds in the hair.
Hydrogen peroxide (H2O2) is the neutralizer that is typically used with thio relaxers to rebuild the disulfide bonds in the hair and stop the chemical process after the hair has been treated.
Thio relaxers work by breaking down the disulfide bonds in the hair, and a neutralizer is used to stop the chemical process and rebuild the disulfide bonds in the hair. Hydrogen peroxide (H2O2) is the neutralizer that is typically used with thio relaxers.
To know more about neutralizer visit
https://brainly.com/question/14156911
#SPJ11
Related Questions
Use the particle theory to explain why 10 mL
of liquid cannot
fill a 20 mL container.
Answers
Answer:
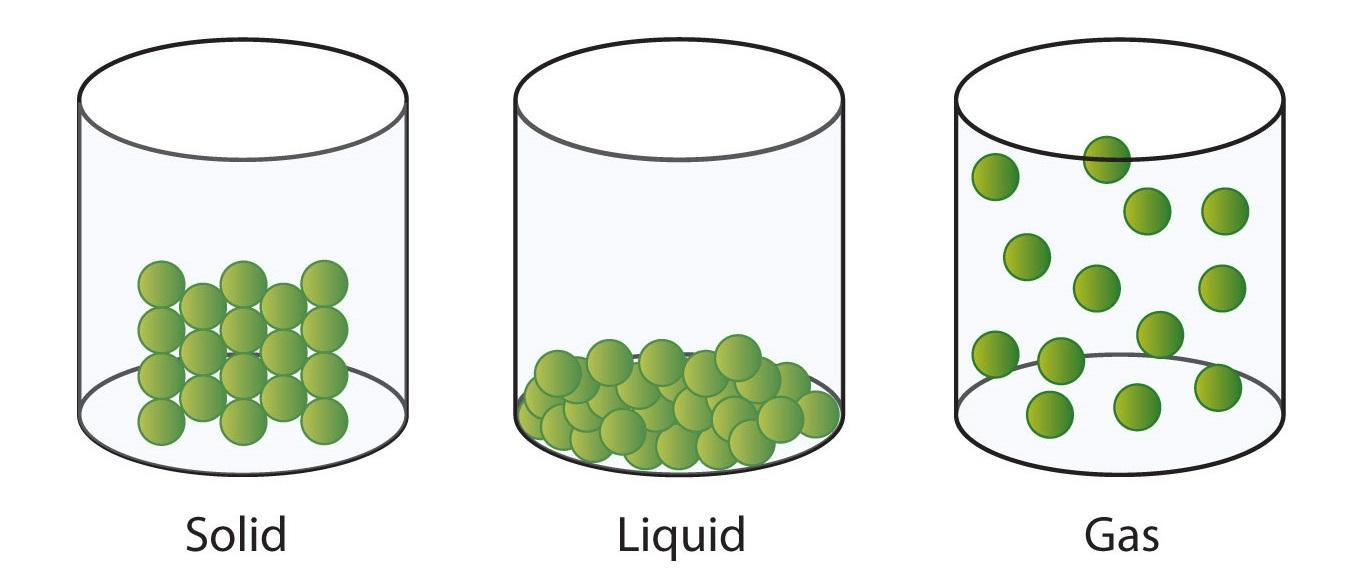
The particle theory is the belief that everything in our solar system and beyond is made of very small matter called atoms. The 10 mL is a matter in our solar system and even though we cannot see, there are millions- if not billions of smaller particles that make up the liquid. If you view the picture below, you can see that the particles in a liquid are close to one another, particles in a gas are far apart, and a particle in a solid is tightly pushed together. This gives them their distinctive shape. Since this is a liquid, this means that the particles are close together, but not very close. The particles glide over one another. If you want to have more space between the particles and expand the size of the liquid you can boil the water, however boiling the water turns it into gas and causing the liquid to evaporate. Freezing the liquid would cause the particles to be closer packed together, making a solid. The amount of liquid you have can not change. There is still 10 mL even when the liquid is frozen or when the liquid boils into vapor in the air. Therefore using particle theory, we can know that a shape can only expand or shrink when changing states of matter.
I hope this helped & Good Luck <3 !!
The three nuclides, U-233, U-235, and U-238, are isotopes of uranium because they have the same number of protons per atom and A) the same number of neutrons per atom B) a different number of electrons per atom C) a different number of neutrons per atom D) the same number of electrons per atom
Answers
Answer:
The correct answer is - C) a different number of neutrons per atom.
Explanation:
Isotopes of an element are the same element and same atomic number but with different atomic mass and physical properties. The difference in their atomic mass occurs due to isotopes of an element have a different number of neutrons per atom.
The number of protons and the numbers of electrons are the same in the isotopes but only change occurs in the numbers of the neutrons. In isotopes of uranium U-233, U-235, and U-238 have the same number of protons but a different number of neutrons per atom.
The three nuclides U-233, U-235, and U-238 are isotopes because they have a different numbers of neutrons. Thus, option C is correct.
Isotopes of an element can be defined as the elements with the same atomic number but different mass numbers. The atomic number can be defined as the number of electrons of an atom.
The isotopes of Uranium thus have the same number of electrons. Thus option B is incorrect.Statement D is correct but is not the characteristic property to differentiate an isotope. Thus, option D is incorrect.The mass number can be defined as the sum of the number of protons and neutrons. The number of electrons and protons is equal in an atom.Thus in an isotope, the number of protons will be the same per atom.
The number of neutrons in an atom has been different per atom. Thus, option A is incorrect. Option C is correct.
Thus the three nuclides U-233, U-235, and U-238 are isotopes because they have a different numbers of neutrons. Thus, option C is correct.
For more information about the isotopes, refer to the link:
https://brainly.com/question/11680817
Shriram saw the Sun on his LEFT while Kabir saw it on his RIGHT.Based on this, Which of the following statements is true?
Answers
Answer:
they were facing the oppisite ways
Explanation:
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
Which statement below best describes the current model of the atom based on Rutherford’s gold foil experiment?
Answers
Answer:
where are the statements??
help please i need it later deadline is later 11:59pm

Answers
Answer:
In the attached image.
Explanation:
DNA to MRNA Thymine get's changed to Uracil. The bases match as:
A- U
U- A
G- C
C- G
Your amino acid is coded from the mRNA

describe one feature that decreases in size between the australopithecus and early members of the homo genus.
Answers
One feature that decreases in size between Australopithecus and early members of the Homo genus is cranial capacity or brain size.
Australopithecus species, such as Australopithecus afarensis, had a smaller cranial capacity compared to early members of the Homo genus, such as Homo habilis. Cranial capacity refers to the volume of the braincase, which is an indicator of brain size. Australopithecus species had an average cranial capacity of about 400-550 cubic centimeters (cc), whereas early Homo species had larger cranial capacities ranging from about 600-800 cc.
The difference in cranial capacity between Australopithecus and early Homo species can be calculated by subtracting the average cranial capacity of Australopithecus (e.g., 500 cc) from the average cranial capacity of early Homo (e.g., 700 cc):
700 cc - 500 cc = 200 cc
The cranial capacity, and thus the brain size, increased between Australopithecus and early members of the Homo genus. This increase in brain size is thought to be associated with the evolution of more advanced cognitive abilities and technological advancements observed in early Homo species compared to Australopithecus.
To know more about Australopithecus visit:
https://brainly.com/question/24650990
#SPJ11
Identify the limiting reactant when 9.65-g H2SO4 reacts with 6.10-g of NaOH.
Answers
Answer:
3.56
Explanation:
41. ) consider the titration of a 35. 0ml sample of 0. 175m hbr with 0. 200m koh. Determine each quantity
Answers
In the titration of a 35.0 mL sample of 0.175 M HBr with 0.200 M KOH, the quantities are approximately 0.006125 moles of HBr and KOH, and 30.6 mL of KOH solution is required for complete reaction.
To determine each quantity in the titration of a 35.0 mL sample of 0.175 M HBr with 0.200 M KOH, we can use the concept of stoichiometry and the equation of the reaction between HBr and KOH:
HBr + KOH → KBr + H₂O
The number of moles of HBr in the 35.0 mL sample can be calculated using the formula:
moles HBr = Molarity * Volume (in liters)
moles HBr = 0.175 mol/L * 0.035 L
moles HBr ≈ 0.006125 mol
Since the balanced equation shows that the ratio between HBr and KOH is 1:1, the number of moles of KOH required for complete reaction is also 0.006125 mol.
The volume of 0.200 M KOH required can be calculated using the formula:
Volume KOH = moles KOH / Molarity
Volume KOH = 0.006125 mol / 0.200 mol/L
Volume KOH ≈ 0.0306 L
Converting the volume to milliliters:
Volume KOH ≈ 30.6 mL
Learn more about titration here:
https://brainly.com/question/29808916
#SPJ11
What is the∆S° of 0₂
Answers
Answer:0
Explanation: zero because it is the most stable form of oxygen in its standard state
Two objects are described below.
Thing 1: It has a volume.
Thing 2: It is made up of particles too small to see.
Which statement is true?
The first object is non-matter, and the second object is matter.
The first object is matter, and the second object is non-matter.
Both objects are matter.
Both objects are non-matter.
Answers
The statement "The first object is non-matter, and the second object is matter" is true.
What is matter?Matter is anything that has mass and takes up space. It is the physical substance that makes up everything around us, including all the objects we can see, touch, and feel.
Matter is composed of tiny particles, such as atoms and molecules, that interact with each other through various forces to create the different forms of matter we observe. Matter exists in different states, including solid, liquid, and gas, depending on the arrangement and motion of its particles.
Thing 1, which has volume, is matter because it occupies space and has mass.
Thing 2, which is made up of particles too small to see, is also matter, as particles are the basic building blocks of matter.
Learn about matter here https://brainly.com/question/16982523
#SPJ1
how many moles are in 1.02 x 10^23 atoms of argon gas
Answers
Answer:
0.17 mol
Explanation:
Given data:
Number of atoms of Ar gas = 1.02×10²³ atom
Number of moles = ?
Solution:
The given problem will solve by using Avogadro number.
The number 6.022 × 10²³ is called Avogadro number.
1 mole = 6.022 × 10²³ atoms
1.02×10²³ atom × 1 mol / 6.022 × 10²³ atoms
0.17 mol
Avogadro number:
It is the number of atoms , ions and molecules in one gram atom of element, one gram molecules of compound and one gram ions of a substance.
How many different elements are involved in the chemical reaction shown
Ca+2H2O>ca(OH)2+H2
Answers
Explanation:
1.ca
2.H
3.O
.................
Which of the following is a characteristic of Byzantine art?
a. Orthogonals
b. Reverse perspective
c. Maniera greca
d. Animal style
Answers
The characteristic of Byzantine art is Maniera greca.
Byzantine art is known for its distinctive style influenced by the Eastern Roman Empire. One of its notable characteristics is the use of "Maniera greca," which refers to the incorporation of elements from Greek art. This influence can be seen in the stylized figures, flat and elongated proportions, and intricate patterns used in Byzantine artwork. Maniera greca also includes the use of gold backgrounds, rich colors, and detailed ornamentation.
These features are commonly found in Byzantine mosaics, icons, and frescoes, reflecting the religious and spiritual nature of the art. This is the correct answer. Maniera greca, or "Greek style," refers to the influence of Greek art and aesthetics on Byzantine art. It is characterized by stylized figures, elongated proportions, intricate patterns, and the use of gold backgrounds. This style was prominent in Byzantine mosaics, icons, and frescoes.
Learn more about Byzantine art here:
https://brainly.com/question/28195718
#SPJ11
Answer please.
Need anwer due in 10 mins.

Answers
Examine the reaction. NH4OH(aq) →H2O(l) + NH3(g)
What coefficients will balance the equation?
A) 1, 1, 1
B) 3, 3, 4
C) 2, 1, 2
D) 1, 2, 2
Answers
Answer: A. 1,1,1
Explanation:
The coefficients that will balance the equation; NH4OH(aq) →H2O(l) + NH3(g), is 1, 1, 1, because it proves the total number of atoms of each element on the LHS and RHS of the equation are equal, hence balanced.
LHS RHS
N = 1 1
H = 5 5
O = 1 1
Calculate the mass percentage composition of nitrogen in acetaminophen, C8H9NO2
Answers
The mass percent composition of nitrogen in acetaminophen is 9.26 %.
The mass percent composition of an element is the percentage of the ratio of the molar mass of that element to the molar mass of the entire compound. Acetaminophen represented as C8H9NO2 is a drug that is used as a pain reliever.
First, we will calculate the molar mass of this compound. For this, we should know the mass of each element present in the compound.
mass of C = 12, mass of H = 1, mas of N = 14, mass of O = 16.
Now, we will calculate the molar mass of acetaminophen
= 12*8+ 1*9+14*1+16*2
= 151 g
Now, we have to calculate the mass percent composition of Nitrogen.
The molar mass of nitrogen = 14g
The molar mass of the entire compound = 151 grams.
Mass percent composition of N = (mm of N ÷ mm of C8H9NO2) ×100
= (14/151) × 100 = 0.0926 × 100
= 9.26 %
Therefore, the mass percent composition of nitrogen in acetaminophen (C8H9N02) is 9.26%.
To learn more about mass percentage composition;
https://brainly.com/question/20393858
How are self heating coffee cans dangerous?
Answers
Answer:
cuz the liquid inside it could get in the machine
Explanation:
Pure water has a boiling point of 100°C and a freezing point of 0°C.
What is the boiling point and freezing point of a sample of aqueous sodium chloride?
A3
А
B
C
D
boiling point/°C
98
98
102
102
freezing point/°C
-2
2
-2
2
Answers
liquid water For example, the limited temperature range of liquid water (0°C–100°C) severely limits its use. Aqueous solutions have both a lower freezing point and a higher boiling point than pure water.
What is temperature ?How hot (or energetic) a substance or radiation is can be quantified by a physical value called temperature.
There are three different types of temperature scales: those like the SI scale that are defined in terms of the average translational kinetic energy per freely moving microscopic particle, like an atom, molecule, or electron in a body; those that only rely on strictly macroscopic properties and thermodynamic principles, like Kelvin's original definition; and those that are defined by actual empirical properties of particulates rather than by theoretical principles.
Temperature is gauged using a thermometer. It is calibrated using a variety of temperature scales that historically defined themselves using various reference points and thermometric materials. The most widely used scale is the Celsius scale, previously called as "centigrade."
To learn more about temperature from the given link:
https://brainly.com/question/24746268
#SPJ4
how much volume in ml will you need to take from 2.7 m concentrated stock solution if you would like to prepare a diluted 0.8 solution with 100 ml? report and round your answer to a whole integer.
Answers
To make a 100 ml solution of 0.8 m from a 2.7 m concentrated stock solution we have to take a volume of: 30 ml of the concentrated solution
To solve this problem, the formula and the procedure that we have to use is:
c1 * v1 = c2 * v2
Where:
c1= concentration of the concentrated solutionv1 = volume of the concentrated solutionc2 = concentration of the diluted solutionv2 = volume of the diluted solutionInformation about the problem:
c1 = 2.7 mv1=?c2 = 0.8 mv2=100 mlApplying the dissolution of concentrations formula and clearing the volume of the concentrated solution (v1) we get:
c1 * v1 = c2 * v2
v1 = (c2 * v2)/ c1
v1 = (0.8 m * 100 ml)/ 2.7 m
v1= 29.6 ml
By rounding the volume to a whole integer value we have:
v1= 30 ml
What is a solution?In chemistry a solution is known as a homogeneous mixture of two or more components called:
Solvent: it usually is in a major amount than the soluteSolute: it usually is in less amount than the solventLearn more about chemical solution at: brainly.com/question/13182946
#SPJ4
what type of reaction is
CuO + H2SO4 ➡️ CuSO4 + H2O
Answers
11) Predict the products of this reaction.
CuSO4(aq) + 2KOH(aq) →
A) Cu(OH)2(aq) + K2SO4(s)
B) Cu(OH)2(s) + K2SO4(aq)
C) CuOH(s) + K2SO4(aq)
D) CuOH2(s) + K2SO4(aq)
Answers
Answer:
6FCRXCTV
Explanation:
ED5RF6GT7HY8JU9KI0LO-;PJH7YNG6GDAnswer:
b
Explanation:
a p e x
Find the number of moles of hydrogen produced from 10.0 moles of aluminum.
2Al + 3H2SO4 --> Al2(SO4)3+3H2
Step to step instructions, please
Answers
Answer:
To find the number of moles of hydrogen produced from 10.0 moles of aluminum, you need to balance the equation for the reaction between aluminum and sulfuric acid.
Step 1: Balance the equation:
2Al + 3H2SO4 --> Al2(SO4)3+3H2
Step 2: Determine the stoichiometric ratio of moles of hydrogen produced per mole of aluminum:
From the balanced equation, 3 moles of hydrogen are produced for every 2 moles of aluminum.
Step 3: Calculate the number of moles of hydrogen produced:
If you start with 10.0 moles of aluminum, then the number of moles of hydrogen produced would be 3 * (10.0 moles of Al / 2 moles of Al) = 15 moles of hydrogen.
Use the information and table to answer the following question.
A group of students collects data to determine whether compounds were ionic or covalent. They placed each
compound in a small bowl and stuck two open ends of a battery-powered circuit in the compound.
Compound
CO2 (s)
KCI (s)
CaO (s)
SIO2 (s)
Conductive
No
No
No
No
Based on the student's data, can they determine which substances are ionic, and which are
covalent?
A. No, as lonic compounds are only conductive in an aqueous (water) solution.
B. Yes, as all of these compounds are lonic since they are not conductive.
C. No, as covalent compounds are only conductive in an aqueous (water) solution.
C
D. Yes, as all of these compounds are covalent since they are not conductive.

Answers
Answer:
d
Explanation
all are compounds
Yes, as all of these compounds are covalent since they are not conductive. Therefore, the correct option is option D.
What is chemical compound?A chemical compound is a material made of several similar molecules (as well as molecular entities) joined by chemical bonds and comprising atoms from various chemical elements.
Chemical reactions, which may entail interactions with other molecules, can change a compound into a distinct substance. Atomic bonds may be shattered or new ones created during this process. Yes, as all of these compounds are covalent since they are not conductive.
Therefore, the correct option is option D.
To know more about chemical compound, here:
https://brainly.com/question/26487468
#SPJ5
________ are weak bonds that are not strong enough to hold atoms together to form molecules but are strong enough to form bonds within and around large molecules.
Answers
Hydrogen bonds are weak bonds that are not strong enough to hold atoms together to form molecules but are strong enough to form bonds within and around large molecules.
The hydrogen bond is weak bond.The hydrogen bond is electrostatic force of attraction between hydrogen atom and more electronegative atoms or group ( like Florine , oxygen or nitrogen) which is contently bonded.The hydrogen bond is occur in polar , contently bond atoms in different molecules.Example is H-O-H or \(NH_{3}\)The positively charged hydrogen side of one water molecule is bond with negatively charged oxygen side of another molecule.learn about Hydrogen bond
https://brainly.com/question/10904296
#SPJ4
.Assertion -SODIUM CHLORIDE is always existed in mixture form. REASON-Substance which made up of more than one constituent called mixture.
Answers
True or false Cell division in Prokaryotes that form two genetically identical cells is know as fission.
Answers
Because Binary fission is the method by which prokaryotes produce new individuals that are genetically identical to the parent organism.
A 100.0 mL sample of 0.18 M HClO 4 is titrated with 0.27 M LiOH. Determine the pH of the solution after the addition of 100.0 mL of LiOH.
12.65
13.13
0.87
12.95
1.35
Answers
The pH of the solution after the addition of 100.0 mL of LiOH is 12.95.
What is pH?pH (potential of Hydrogen) is a measure of the acidity or alkalinity of a solution, with a value of 7 being neutral. It is measured on a logarithmic scale from 0 to 14, with 0 being the most acidic and 14 being the most alkaline. Solutions with a pH lower than 7 are considered acidic and solutions with a pH higher than 7 are considered alkaline.
The pH of the solution after the addition of 100.0 mL of LiOH can be calculated using the Henderson-Hasselbalch equation.
pH = pKa + log([base]/[acid])
Where pKa is the acid dissociation constant for HClO₄, which is 3.45, and [base] and [acid] are the concentrations of the base (LiOH) and acid (HClO₄), respectively.
Plugging in the values, we get:
pH = 3.45 + log(0.27/0.18)
pH = 12.95
Therefore, the pH of the solution after the addition of 100.0 mL of LiOH is 12.95.
To learn more about pH
https://brainly.com/question/172153
#SPJ4
help me please help me help me help me help me

Answers
The cell supplies the potential energy
The bulbs become weaker
The brightness of the bulbs will increase
What happens when more bulbs are connected in series?In a simple series circuit, there are two components connected in series, such as a resistor and a battery. When a voltage is applied across the circuit, the current flows through the resistor and then through the battery, in a continuous loop.
The voltage across the resistor is proportional to the current and the resistance of the resistor, according to Ohm's law, while the voltage across the battery remains constant.
Learn more about series connection:https://brainly.com/question/18713901
#SPJ1
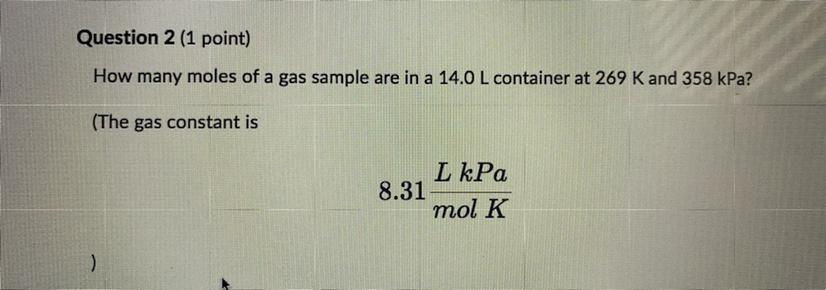
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles