When 85 L of gas at 1.376 atm has its pressure changed to 0.154 atm, what is the new volume?
Answers
Answer:
759.48 LExplanation:
The new volume can be found by using the formula for Boyle's law which is
\(P_1V_1 = P_2V_2\)
Since we are finding the new volume
\(V_2 = \frac{P_1V_1}{P_2} \\\)
From the question we have
\(V_2 = \frac{85 \times 1.376}{0.154} = \frac{116.96}{0.154} \\ = 759.480519...\)
We have the final answer as
759.48 LHope this helps you
Related Questions
Sodium bicarbonate is the chemical name for baking soda. Its chemical formula is NaHCO3. What does the subscript 3 mean?
Answers
Answer:
'3' in NaHCO₃ is the number of moles oxygen in 1 mole of sodium bicarbonate
Explanation:
see above note.
C.
There Are Three Atoms Of Oxygen In Sodium Bicarbonate.
_______
I Got It Right On The Test, Hope It Helps :)
Which substance, when mixed with water, will produce the best conductor of
electricity?
a) table salt
b) granulated sugar
c) carbon dioxide
d) motor oil
Answers
Answer: a) Table Salt
good luck
How many moles of calcium carbonate are in 63.8 g of calcium carbonate?
Answers
Answer:
0.638 moles in 63.8g of calcium carbonate
Explanation:
possible isomers of C4H9I
Answers
Answer:
there are 4 possible isomers
according to molecular orbital theory, where is the charge of the allyl carbocation below associated?
Answers
The delocalization energy as the difference between the total energy of the π electrons and the energies of the individual π bonds: an electron in one π bond has the energy of α+β.
MO theory and Resonance
- in MO theory, the entire molecule is treated as one entity, and all of the electrons in the entire molecule occupy regions of space called molecular orbitals.
- Two electrons are placed in each orbital, starting with the lowest energy orbital, until all electrons occupy orbitals.
- According to MO theory, the three p orbitals no longer exist. Instead, they have been replaced by three MOs, in order of increasing energy. Notice that the lowest energy MO, called the bonding molecular orbital, has no vertical nodes. The next higher energy MO, called the nonbonding molecular orbital, has one vertical node. The highest energy MO, called the antibonding molecular orbital, has two vertical nodes.
- The π electrons of the allyl system will fill these MOs, starting with the lowest energy MO.The allyl carbocation has only two π electrons, rather than three, because one of the carbon atoms bears a positive formal charge indicating that one electron is missing. The two π electrons of the allyl system will occupy the lowest energy MO (the bonding MO). If the missing electron were to return, it would occupy the next higher energy MO, which is the nonbonding MO. Focus your attention on the nonbonding MO.
There should be an electron occupying this nonbonding MO, but the electron is missing. Therefore, the colored lobes are empty and represent regions of space that are electron deficient. In conclusion, MO theory suggests that the positive charge of the allyl carbocation is associated with the two ends of the system, rather than just one end.
To know more about Resonance :-
brainly.com/question/29547999
#SPJ4
How much hydrogen reacts with 32 g of oxygen to produce 34 g of hydrogen peroxide. help and thxsss so much
Answers
Answer: the answer is 2g because you subtract 32-34 and get 2 and add the get so 2g
Explanation:
2 g of hydrogen reacts with 32 g of oxygen to produce 34 g of hydrogen peroxide.
To determine the amount of hydrogen that reacts with 32 g of oxygen to produce 34 g of hydrogen peroxide (\(H_2O_2\)), we need to apply stoichiometry.
First, we find the molar masses:
Molar mass of oxygen (\(O_2\)) = 16 g/mol
Molar mass of hydrogen peroxide (\(H_2O_2\)) = 34 g/mol (given)
Next, we calculate the number of moles for oxygen and hydrogen peroxide:
Moles of oxygen = mass/molar mass = 32 g / 16 g/mol = 2 moles
Moles of hydrogen peroxide = mass/molar mass = 34 g / 34 g/mol = 1 mole
Since hydrogen peroxide has two hydrogen atoms, it requires twice the moles of hydrogen. Therefore, the moles of hydrogen required are also 2 moles.
Finally, we convert the moles of hydrogen to grams:
Mass of hydrogen = moles × molar mass = 2 moles × 1 g/mol = 2 g
So, 2 g of hydrogen reacts with 32 g of oxygen to produce 34 g of hydrogen peroxide
To know more about hydrogen, here
brainly.com/question/30623765
#SPJ2
Describe how to make 200.0 mL of 50.0 mM NaOH starting from a 2.50
M NaOH stock. Include all weights/volumes, measuring devices, and
labware.
Answers
To make 200.0 mL of 50.0 mM NaOH starting from a 2.50 M NaOH stock, you need to follow these steps: calculations and preparation is the answer.
Step 1: Calculation- First, you need to calculate the amount of 2.50 M NaOH solution needed to make 200.0 mL of 50.0 mM NaOH.
The molar mass of NaOH = 40 g/mol
Molarity = moles of solute/litres of solution50.0 mM NaOH = 50.0 × 10⁻³ moles/L.
Let's assume that we need x mL of 2.50 M NaOH.
Then the number of moles of NaOH is given by: Moles of NaOH in x mL of 2.50 M solution = 2.50 × 10⁻³ moles/mL × x mL
Now, equate the number of moles of NaOH in the 2.50 M solution to the number of moles of NaOH in the 50.0 mM solution:2.50 × 10⁻³ moles/mL × x mL = 50.0 × 10⁻³ moles/L × 0.200 L∴ x = 4.00 mL.
So, you need 4.00 mL of 2.50 M NaOH to make 200.0 mL of 50.0 mM NaOH solution.
Step 2: Preparation -Now, take 4.00 mL of 2.50 M NaOH stock solution in a clean beaker.
Add distilled water to make the volume up to 200.0 mL.
Stir the solution with a clean glass rod to ensure uniformity. Store the solution in a clean, labelled reagent bottle.
know more about molar mass
https://brainly.com/question/31545539
#SPJ11
Please help, I'll give brainiest
Coffee is a solution containing caffeine, acids, alkaloids, water, phenols, and many other chemicals. The solvent in the coffee solution is (1) __________ and the rest of the chemicals dissolved in the solvent are called (2)_________.
3. What is the difference between a saturated solution and a supersaturated solution?
4. Is the following molecule, polar or nonpolar?
5. 12 eggs can be referred to as one dozen eggs.
______________ particles can be referred to as one mole of particles.
6.
The pH of a strong acid might be _____ while the pH of a strong base might be _____
The pH of pure water is _____.
7. What would be the pH of .002 moles of HNO3 dissolved in 2 L of water?
8. A base has a pH of 8.5. What is the concentration of OH- ions in the solution?
Answers
Solutes dissolve in solvents to form a solution. A saturated solution contains just as must solute as it can normally hold.
The solvent in the coffee solution is water and the rest of the chemicals dissolved in the solvent are called solutes. A solution is formed when a solute is dissolved in a solvent.
A saturated solution contains just as much solute as it can normally hold at a particular temperature while a supersaturated solution contains more solute than it can normally hold at a particular temperature.
A polar molecule contains covalent bonds between atoms having an electronegativity difference above 0.5. Such molecules are polar as electrons of the bond are drawn closer to the atom that is more electronegative.
According to Avogadro's law; 6.02 × 10^23 particles is referred to as one mole of particles.
A strong acid has a pH that may range from 0 - 3. A strong base has a pH of around 10 - 14. Water is a neutral substance and has a pH of 7.
From the information provided;
Number of moles of acid = 0.002 moles
Volume of solution= 2 L
Concentration of solution = number of moles/volume = 0.002 moles/2L = 0.001 M
pH = - log[H^+]
pH = - log[0.001 ]
pH = 3
From;
pH + pOH = 14
pOH = 14 - pH
pOH = 14 - 8.5
pOH = 5.5
pOH = - log[OH^-]
[OH^-] = Antilog[-5.5]
[OH^-] = 3.2 × 10^-6 M
Learn more: https://brainly.com/question/10609459
How many moles are in 64 g of O2?
Answers
Answer:
4 moles
Explanation:
Thus, 64 grams of oxygen contains 4 moles.
Hold the slides for observation
Answers
Explanation
Which elements will form cations and which will form anions

Answers
Sodium losses electrons to become Na+, thus forms a cation.
Fluoride gains electrons to become F-, thus forms an anion.
Iron losses electrons to become Fe2+, thus forms a cation.
Silver also losses electrons to be Ag+, thus forms a cation.
Newtons third law explains what happens when two objects-
Answers
Answer:
When one object uses force to move the other.
Explanation:
This is called Thrust.Thrust is used in airplane engines,or an engine can be a source of Thrust,we use our legs to Thrust us forward when we walk!
Sir Isaac Newton's third law explains that for every action, or force, in nature, there is an equal and opposite reaction.
Hope that helps!
0. A radioactive isotope has a half-life of 273 days. How much of a sample of 100 grams of the isotope would remain after 732 days?
Answers
The amount of a sample of 100 grams of a radioactive isotope that would remain after 732 days would be 14.0625 grams.
Given, the Half-life of the radioactive isotope = 273 days.Time elapsed = 732 days.Initial quantity or sample = 100 grams. Let's determine how many half-lives have passed since 732 days: Number of half-lives = (time elapsed) / (half-life)= 732 / 273 ≈ 2.683
Half-life #1: After the first half-life of 273 days, the sample will be halved. Therefore, after 273 days, the quantity remaining will be 1/2 * 100g = 50g
Half-life #2: After the second half-life of 273 days, the sample will be halved again. Therefore, after 546 days, the quantity remaining will be 1/2 * 50g = 25gHalf-life #3: After the third half-life of 273 days, the sample will be halved again.
Therefore, after 819 days, the quantity remaining will be 1/2 * 25g = 12.5gHowever, the time elapsed from 819 days to 732 days is 87 days. This time interval is less than the half-life. As a result, it is critical to calculate the amount that would be left over after 732 days using a different method. Let us consider the remaining amount from 819 days (12.5g) as the new initial quantity for the remaining 87 days. The half-life of the radioactive isotope is 273 days.
Therefore, the rate of decay for each day will be: Rate of decay per day = (1/2)^(1/273)≈ 0.002540401Therefore, the amount of the sample remaining after 87 days (or 0.3195 half-lives) can be calculated using the following formula: Q = Q0(0.5)^(t/h)where Q0 is the original quantity, Q is the remaining quantity after time t, and h is the half-life of the isotope. Q = 12.5g × (0.5)^(0.3195)Q ≈ 6.5625g
Therefore, the total amount of the sample remaining after 732 days can be found by adding up the amounts of the sample remaining from each half-life: Total remaining = 50g + 25g + 6.5625gTotal remaining ≈ 81.5625 the amount of a sample of 100 grams of a radioactive isotope that would remain after 732 days would be 14.0625 grams.
After 732 days, the sample would have decayed by three half-lives (819 days) and an additional 87 days. As a result, 81.5625g of the sample will remain after 732 days. Therefore, 100g - 81.5625g = 18.4375g of the sample would have decayed in 732 days.
To know more about isotopes, visit:
https://brainly.com/question/14220416
#SPJ11
7
When one kind of cell changes to a specialized cell, this is an example of ... (S
Answers
Answer:
Cellular differentiation
Explanation:
Answer:
cellular differentiation
Explanation:
Where are the electrons inside an atom?
A.The electrons are in the nucleus of an atom
B.They are arranged in energy levels
C.The electrons are spread equally throughout the atom
Answers
Answer: B.They are arranged in energy levels
Explanation: Electrons orbit around the nucleus of an atom, and are not spread around equally throughout an atom because there are a different amount of electrons that can fit in each energy level or electron shell (both are the same thing). For example the first energy level can hold up to 2 electrons, the second shell can hold up to 8 electrons, the third can hold up to 18, and so on represented by the equation 2(n^2)
if a bar of motilium cost .0.2 dollars how much will 200 bars cost
Answers
Answer: 40
Explanation: 0.2 x 200
What is the answer for:
C8H18+Br2=
Answers
2(72ch+br)
that's the answer
Using the regulatory portions of the lac operon as an example, explain the difference between cis and trans acting regulatory factors.
Answers
The lac operon is a classic example of gene regulation in bacteria. It consists of regulatory elements that control the expression of the lac genes involved in lactose metabolism. The lac operon includes both cis-acting and trans-acting regulatory factors.
Cis-acting regulatory elements refer to DNA sequences that are located on the same DNA molecule as the genes they regulate. In the case of the lac operon, the cis-acting element is the lac operator (lacO), which is situated adjacent to the lac genes. The lacO serves as a binding site for the lac repressor protein (encoded by the lacI gene) and plays a role in controlling the accessibility of the lac genes for transcription.
Trans-acting regulatory factors, on the other hand, are regulatory molecules or proteins that are produced elsewhere in the cell and act on the cis-acting elements. In the lac operon, the lac repressor protein (encoded by the lacI gene) is a trans-acting factor. It can bind to the lac operator (cis-acting element) and inhibit the transcription of the lac genes when lactose is absent or in low concentrations.
Learn more about lac operon here:
https://brainly.com/question/2562849
#SPJ11
The reaction of CaO and water isexothermic. A student mixes the twochemicals in a test tube and touchesthe side of the test tube. Whichstatement describes the student’sobservation? A The test tube becomes hot as heatis released. B The test tube becomes hot as heatis absorbed. C The test tube becomes cold asheat is released. D The test tube becomes cold asheat is absorbed.
Answers
If a student mixes these two chemicals in a test tube and touches the side of the test tube, they will observe a change in temperature. The test tube becomes hot as heat is released. The correct option is A.
When CaO and water react, they produce calcium hydroxide and release heat, making the reaction exothermic. If a student mixes these two chemicals in a test tube and touches the side of the test tube, they will observe a change in temperature. The question asks which statement best describes the student's observation.
The correct answer would be A: The test tube becomes hot as heat is released. This is because the reaction between CaO and water is exothermic, meaning that it releases heat into the surrounding environment. Therefore, the test tube will become hot as a result of the heat being released.
Option B, "The test tube becomes hot as heat is absorbed," is incorrect because an exothermic reaction releases heat, rather than absorbing it. Option C, "The test tube becomes cold as heat is released," is also incorrect because the reaction releases heat, causing the test tube to become hotter. Option D, "The test tube becomes cold as heat is absorbed," is incorrect because, again, an exothermic reaction releases heat rather than absorbing it.
In summary, the correct observation would be that the test tube becomes hot as heat is released due to the exothermic reaction between CaO and water. The correct option is A.
To know more about exothermic reaction, refer to the link below:
https://brainly.com/question/28546817#
#SPJ11
An object has a mass of 183.5 g and a density of 14.8 g/cm³. Determine the volume of the objectin cm³.
Answers
First, let's remember the formula to calculate an object's density:
\(\begin{gathered} \rho=\text{ }\frac{m}{V} \\ \\ Being\text{ }\rho\text{ the density, m the mass, and V the volume.} \end{gathered}\)Then, we analyze what we have:
\(\begin{gathered} m\text{ = 183.5 g} \\ \rho=\text{ 14.8 g/cm}^3 \end{gathered}\)We need to determine the volume, so we transform our formula like this:
\(V=\text{ }\frac{m}{\rho}\)We replace our data:
\(V=\text{ }\frac{183.5\text{ g}}{14.8\text{ g/cm}^3}=\text{ 12.399 cm}^3\approx\text{ 12.4 cm}^3\)Then, the answer is that the volume equals 12.4 cm^3.
Ethyl trichloroacetate is significantly more reactive toward hydrolysis than ethyl acetate. Explain this observation.
Answers
Ethyl trichloro acetate is significantly more reactive toward hydrolysis than ethyl acetate because of the polar nature of the functional group. Ethyl trichloro acetate is more reactive towards hydrolysis than ethyl acetate because of the polar nature of the functional group.
The reaction between an ester and water is known as hydrolysis. An ester is a derivative of carboxylic acid, which contains a carbonyl group that is linked to an alkyl or an aryl group through an oxygen atom. The hydrolysis of esters involves the addition of water to the ester's carbonyl group, resulting in the formation of a carboxylic acid and an alcohol.The reactivity of esters toward hydrolysis is determined by the alkyl or aryl group linked to the carbonyl group, as well as the functional group. The higher the electron density of the carbonyl group, the more reactive the ester will be toward hydrolysis.
Ethyl trichloro acetate has a more polar carbonyl group than ethyl acetate. Trichloro acetate has a chlorine atom that draws electron density away from the carbonyl group, reducing the electron density of the carbonyl group, making it more electrophilic and reactive towards nucleophiles like water. Therefore, ethyl trichloro acetate is significantly more reactive towards hydrolysis than ethyl acetate.
To know more about Ethyl trichloro acetate refer to:
https://brainly.com/question/16181011
#SPJ11
now you will investigate the emission spectra for a different element, helium. helium is the next element after hydrogen on the periodic table and has two electrons. do you think the emission spectra for an atom with two electrons instead of one will be significantly different than that of hydrogen? explain your answer.
Answers
The electron configuration of Helium (He) is 1s², which means that it has two electrons in its outermost shell.
Helium is an inert gas and, like hydrogen, it also emits a line spectrum when it is energized.Helium has a more complex spectrum than hydrogen because it has more electrons.
As a result, it emits more lines than hydrogen. Helium has two electrons, which implies that it will have twice the number of lines than hydrogen.
The emission spectrum of helium will have more lines than that of hydrogen because helium has more electrons.
To know more about electron configuration click on below link:
https://brainly.com/question/29757010#
#SPJ11
Calculate the new volume if in a container there is a mass of gas that occupies a volume of 1.3L at a temperature of 280K. Calculate the volume when reaching a temperature of 303K.
Answers
Answer:
1.4 L
Explanation:
From the question given above, the following data were obtained:
Initial volume (V1) = 1.3 L
Initial temperature (T1) = 280 K
Final temperature (T2) = 303 K
Final volume (V2) =?
With the above data, we can obtain the new volume of the gas by using the Charles' law equation as shown below:
V1 /T1 = V2 /T2
1.3/280 = V2 /303
Cross multiply
280 × V2 = 1.3 × 303
280 × V2 = 393.9
Divide both side by 280
V2 = 393.9/280
V2 = 1.4 L
Thus, the new volume of the gas is 1.4 L
How many molecules in 3.5 moles in NaCl
Answers
The number of molecules in 3.5 moles of NaCl is 2.107 × 10²⁴ molecules.
How to calculate molecules?The number of molecules in a substance can be calculated by multiplying the number of moles by Avogadro's number as follows:
no of molecules = no of moles × 6.02 × 10²³
According to this question, 3.5 moles of NaCl are given. The number of molecules can be calculated as follows:
no of molecules = 3.5 × 6.02 × 10²³
no of molecules = 2.107 × 10²⁴ molecules
Therefore, 2.107 × 10²⁴ molecules is the number of molecules in the NaCl.
Learn more about no of molecules at: https://brainly.com/question/28319191
#SPJ1
CaC2 + 2H2O → C2H2 + Ca(OH)2If 4.8 moles of CaC2 are consumed in this reaction, how many grams of H2O are needed?
Answers
The given reaction is already balanced, that is to say tha the number of atoms in the reactants matches the number of atoms in the products. In the reaction, we can see the relationship between CaC2 and H2O. For each mole of CaC2 two moles of H2O react.
So, if 4.8 moles of CaC2 are consumed the moles of H2O needed will be:
Mol of H2O = Mol of CaC2 x 2
Mol of H2O = 4.8 x 2 = 9.6 mol of H2O
Now, to calculate the grams of H2O we will use the following equation and the mass molar of H2O.
Mass molar of H2O =18.01 g/mol
\(\begin{gathered} \text{Mass of H2O=Mol of H2O }\times Mass\text{ molar of H2O} \\ \text{Mass of H2O = 9.6 mol }\times18.01\frac{\text{ g}}{mol} \\ \text{Mass of H2O = 172.9 g} \end{gathered}\)So, if 4.8 moles of CaC2 are consumed in this reaction, 172.9 g of H2O are needed
how can you blance it and make it equal on both sides
2H2+o2=2H2o blance it
Answers
Answer:
it have been already balanced
2H2 + O2 = 2H2O.
what is a ligand? select the correct answer below: an atom, molecule, or ion that can form a covalent bond to a metal atom by donating electrons an atom, molecule, or ion that can form a covalent bond to a metal atom by accepting electrons an atom, molecule, or ion that can form an ionic a bond to a metal atom through strong electrostatic interactions an atom, molecule, or ion that can form a covalent bond to a metal atom by sharing electrons
Answers
Ligand is described as an atom, molecule, or ion that can form an ionic a bond to a metal atom through strong electrostatic interactions, option C.
A metal ion in solution doesn't exist by itself; instead, it forms complex ions or coordination compounds with ligands (such as solvent molecules or other simple ions) or chelating groups. These complexes have a core atom or ion, frequently a transition metal, and an encircling collection of ions or neutral molecules. Ions or neutral molecules known as ligands form bonds with a central metal atom or ion. The core atom functions as a Lewis acid (electron pair acceptor), whereas ligands operate as Lewis bases (electron pair donors). In order to create covalent connections with the central atom, ligands must have at least one donor atom with an electron pair.
Alfred Stock introduced the term "ligand" for the first time in reference to silicon chemistry in 1916. The phrase is derived from the Latin word "ligare," which means "to bind." Anions, cations, or neutral substances can serve as ligands. The concept of teeth (dent) is introduced in the classification of ligands as monodentate, bidentate, tridentate, etc., leading to the concepts of biting angle, etc. A monodentate ligand uses a single donor atom to form a connection with the metal atom or ion at its centre.
Learn more about Ligand:
https://brainly.com/question/30438388
#SPJ4
Which compound contains ionic bonds?
1) N2O
2) Na2O
3) CO
4) CO2
Answers
Answer:
The ionic bond present between CaO molecule
Explanation:
In the laboratory you dilute 3.91 mL of a concentrated 3.00 M perchloric acid solution to a total volume of 100 mL. What is the concentration of the dilute solution
Answers
The concentration of the dilute solution is 0.1173 M. To find the concentration of the dilute solution, we can use the formula for dilution:
C1V1 = C2V2
In the above formula:
C1 = Concentration of the concentrated solution
V1 = Volume of the concentrated solution
C2 = Concentration of the dilute solution
V2 = Total volume of the dilute solution
In this case:
C1 = 3.00 M
V1 = 3.91 mL = 0.00391 L
V2 = 100 mL = 0.1 L
Now, let's calculate the concentration of the dilute solution (C2):
C1V1 = C2V2
(3.00 M)(0.00391 L) = C2(0.1 L)
0.01173 mol = 0.1 C2
C2 = 0.01173 mol / 0.1 L
C2 = 0.1173 M
As a result, the diluted solution has a concentration of 0.1173 M.
To learn more about concentration, visit:
https://brainly.com/question/3045247
#SPJ11
someone help me please....
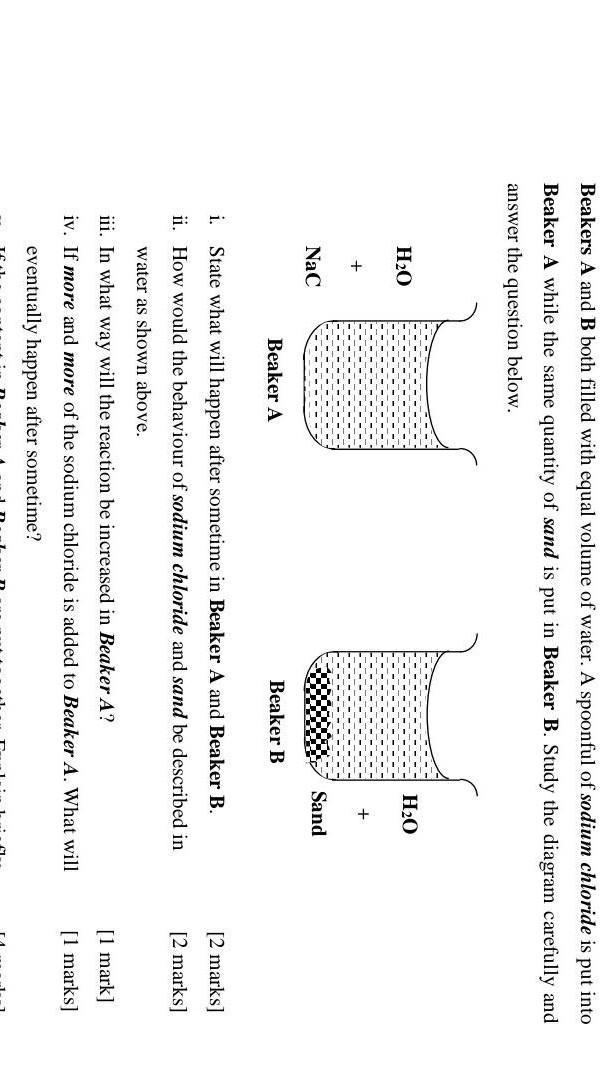
Answers
Answer:
i- In beaker A, sodium chloride will dissolve with water.
and in beaker B, there will be no reaction.
ii- sodium chloride is soluble in water, while sand is insoluble.
iii- The reaction can be increased by adding more sodium chloride to the beaker.
iv- The sodium chloride will no longer dissolve in the water.