Answers
Most liquids expand in volume when the temperature rises, which causes the density of the liquids to decrease. Similar to this, most liquids experience volume loss when temperature decreases, which raises density.
What density of the liquid sample depend on temperature?The density of a liquid is determined by the mass, size, and closeness of the packing of its molecules. Like a solid, the mass of a liquid divided by its volume is how dense it is.
As heat is supplied, liquids expand, and when pressure is applied, they condense. This means that while fluid density increases with pressure, it decreases as temperature rises.
The sample's density decreases and its volume increases. The relationship between a liquid sample's density and temperature is inverse.
Therefore, A liquid sample's density reduces as temperature rises. This is due to the fact that as heat is introduced to the sample, the molecules' kinetic energy and vibration both increase.
Learn more about density here:
https://brainly.com/question/29775886
#SPJ1
Related Questions
A 5 kg ball is traveling at the same speed as a 10 kg ball. Compared to with 5 kg ball, the 10 kg ball has (2 points)
Answers
Answer: twice the momentum
Explanation:
How moles of Cl' are in a 45 mL of 1,4 M solution of NaCl?
Answers
Answer:
.063 moles Cl-
Explanation:
To find moles multiply molarity by volume.
1.4*.045=.063 moles Cl-
What is an example of a nonrenewable resource? (2 points)
a
Oil
b
Sunlight
c
Water
d
Wind
Answers
The answer is A: Oil.
Answer:
Explanation:
A. Oil
hope it helps!
How many moles of H2O are found in a sample containing 7.1 * 10 (19) molecules
Answers
The sample containing 7.1 × 10^19 molecules of H2O corresponds to approximately 1.18 × 10^(-4) moles of H2O.
To determine the number of moles of H2O in a sample containing 7.1 × 10^19 molecules, we need to use Avogadro's number, which states that 1 mole of any substance contains 6.022 × 10^23 molecules.
Given that there are 7.1 × 10^19 molecules of H2O in the sample, we can calculate the number of moles using the following formula:
Moles = Number of molecules / Avogadro's number
Moles = 7.1 × 10^19 / 6.022 × 10^23
Moles ≈ 1.18 × 10^(-4) moles
For more such questions on molecules
https://brainly.com/question/24191825
#SPJ8
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
A particular reaction has a DH o value of -164 kJ and DS o of -185 J/K at 298 K. Calculate DG o at 617 K in kJ (with 3 significant digits), assuming that DH o and DS o hardly change with temperature
Answers
Answer:
e
Explanation:
what's another name for potential energy?
1. kinetic
2. stored
3. mechanical
4. moving
Answers
Answer: Stored energy
Explanation:
Answer:
2. stored energy It is 100% right
Explanation:
Which experiment led to the idea that atoms contained a nucleus?
O A. Niels Bohr's photoelectric effect experiment
O B. Ernest Rutherford's gold foil experiment
O C. J. J. Thomson's cathode ray experiment
O D. Robert Millikan's oil drop experiment
Answers
Answer:
B. Ernest Rutherford's gold foil experiment
Explanation:
That experiment showed a space with the tiny dense spot, referred to as the nucleus. The experiment involved shooting alpha particles (helium nuclei) at a very thin sheet of gold and then observing.
Answer ASAP please ! When sodium (Na) and chlorine (Cl) are combined, a small amount of table salt is formed and a bright yellow light and a lot of heat are given off. Does it take more energy to break the bonds of the reactants, or is more energy released when forming the bonds of the products? How do you know?
a. More energy is released when forming the bonds of the products because a lot of heat is given off, which indicates energy was released.
More energy is released when forming the bonds of the products because a lot of heat is given off, which indicates energy was released.
b. It takes more energy to break the bonds of the reactants because yellow light is given off, which indicates energy was absorbed in the beginning.
It takes more energy to break the bonds of the reactants because yellow light is given off, which indicates energy was absorbed in the beginning.
c. It takes more energy to break the bonds of the reactants because a lot of heat is given off, which indicates energy was absorbed in the beginning.
It takes more energy to break the bonds of the reactants because a lot of heat is given off, which indicates energy was absorbed in the beginning.
d. More energy is released when forming the bonds of the products because yellow light is given off, which indicates energy was released.
More energy is released when forming the bonds of the products because yellow light is given off, which indicates energy was released.
Answers
Answer:
More energy is released when forming the bonds of the products because a lot of heat is given off, which indicates energy was released.
Explanation:
The heat energy is a better indicator of how much energy is used and where.
Which of the following is a good practice to aid in preventing spillage?
Answers
There are many ways in which it can be ensured that chemicals do not get spilled .
To store Chemicals in Covered AreasTo use Spill Kits, Bunds, and Spill PalletsTo store Containers on Secure ShelvingTo prevent Overcrowding in Chemical Storage UnitsTo ensure Chemicals Are Stored at or Below Eye LevelTo regularly Inspect Chemical Containers on Site for Leaks or DeteriorationTo safeguard the Transportation of Chemical ContainersTo implement Strict Decanting Procedures.How quickly a chemical spill or leak can cause a serious accident or disaster is shocking. Chemical production, storage, and transportation all present a multitude of potential accident sites due to their volatile nature. Chemical safety in the workplace can be greatly increased by putting in place a strong emergency response plan and well-established spill prevention procedures.
To know more about chemicals safety, please refer:
https://brainly.com/question/26358183
#SPJ4
How many significant digits are in this number: 0.0030670?
Answers
Answer:
Answer:
Number of Significant Figures: 5
The Significant Figures are 3 0 6 7 0
Explanation:
hope this helps
Duncan takes a break from studying and goes to the gym to swim laps if swimming burns, 615,000 cal per hour, how many kilojoules does swimming burn in the same amount of time?
Answers
which are made matters
Answers
Answer:
Everything you can hold, taste, or smell is made of matter. Matter makes up everything you can see, including clothes, water, food, plants, and animals. It even makes up some things you cannot see, such as air or the smell of perfume.
Which of the following will decrease the pressure of a gas in a closed container?
A. increasing the number of molecules
B. increasing the amount of light
C. increasing the temperature
D. increasing the volume
please answer asap thank you :))
Answers
Now gimmie brainliest
Increasing the number of molecules will decrease the pressure of a gas in a closed container. Hence, option A is correct.
What is pressure?Pressure, in the physical sciences, the perpendicular force per unit area, or the stress at a point within a confined fluid.
An increase in the number of gas particles in the container increases the
frequency of collisions with the walls and therefore the pressure of the gas.
If the temperature decreases the pressure will also decrease.
Temperature is a measure of kinetic energy. When the temperature inside the container decreases the kinetic energy will also decrease.
Hence, option A is correct.
Learn more about the pressure here:
https://brainly.com/question/356585
#SPJ2
Which set of terms best defines what affects kinetic energy and potential energy, respecrively
Answers
helppp nowwww plsss

Answers
Explanation:
The River rushing down through the bottom of the canyon would be your answer but if not then you can try The Rocks falling due to the gravity :)
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
3. A solution of hydrochloric acid is made by dissolving hydrogen chloride gas in 100.0ml water. This solution neutralizes a 15ml sample of 0.10 mol/L sodium carbonate solution. a. What mass of hydrogen chloride gas was dissolved in 100.0ml of water? b. What volume of hydrogen chloride was this?
Answers
Okay, here are the steps to solve this problem:
a) To neutralize 15ml of 0.10 mol/L sodium carbonate solution, the hydrochloric acid solution must contain 0.015 moles of HCl.
Since the HCl solution is made by dissolving HCl gas in 100ml water, we can calculate the moles of HCl gas dissolved in 100ml:
0.015 moles HCl / 15ml sodium carbonate solution = X moles HCl gas / 100ml HCl solution
X = 0.015 * (100/15) = 0.01 moles HCl gas
b) Molar volume of HCl gas at STP is 24.45 L/mol.
So the volume of 0.01 moles HCl gas is: 0.01 moles * 24.45 L/mol = 0.2445 L
Since the solution is made with this gas in 100ml water, the volume of HCl gas dissolved in 100ml water is 0.2445 L.
So the final answers are:
a) 0.01 moles of HCl gas
b) 0.2445 L of HCl gas
Please let me know if you have any other questions!
consider the balanced chemical equation below. when the chemical reaction was carried out calculated theoretical was yield for sodium bromide 162 grams but the measured yield was 150 grams what is the percent yield?
Answers
Answer:
Explanation:
% yield = (actual yield / theoretical yield) X 100
For this question,
% yield = (150g/ 162 g) X 100 = 92.6%
Answer:
92.6%
Explanation:
How many molecules are in 41.5 moles of CO₂?
Answers
41.5 moles CO₂ * 6.022 x 10^23 molecules/mole = 248,839,500,000,000,000 molecules CO₂
So there are approximately 248,839,500,000,000,000 molecules of CO₂ in 41.5 moles of CO₂.
during a baseball game the sound of the bat hitting the ball
Answers
During a baseball game, the sound of the bat hitting the ball can be heard in most parts of the stadium. That sound is weaker at greater distances. The cause of this phenomenon is inverse-square law.
What is sound?Sound is a physical disturbance from an equilibrium condition that travels via an elastoplastic medium. A completely subjective definition of sound, as perceived by the ear, is also viable, but it is not especially informative and is overly limited, because it is important to speak of noises which can be heard by the auditory system, such as those produced by dog whistles or sonar technology.
The inverse-square law, that states that the strength of a sound wave is inversely related to the square of the distance to the source, is the cause of this phenomena. The strength of such sound wave reduces as the distance to the source rises.
Therefore, the cause of this phenomenon is inverse-square law.
To learn more about sound, here:
https://brainly.com/question/733324
#SPJ1
The atomic mass of an element is:
A. The number of protons and electrons
B. The number of protons and neutrons
Answers
Explanation:
it b
....
hope it helps!
7. Which of the following is not a characteristic of a solution?
a. It is a uniform mixture
b. It will scatter a beam of light
c. The solute and solvent cannot be distinguished by the naked eye
d. The solute particles cannot be separated by filtering
Answers
Hopeful it help
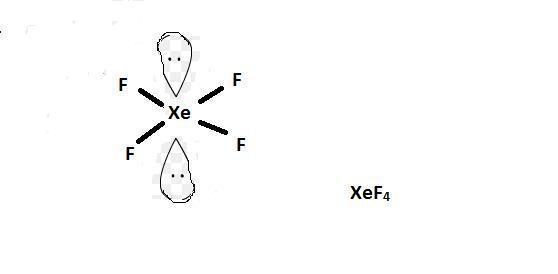
Xenon tetrafluoride has two sets of lone pairs of electrons. What should be the relative positions of the two sets of lone pairs?Why?
Answers
Answer: Two equatorial positions so as to minimize the repulsion.
Explanation:
Formula used for calculating number of electrons :
\(\frac{1}{2}[V+N-C+A]\)
where,
V = number of valence electrons present in central atom = 8
N = number of monovalent atoms bonded to central atom = 4
C = charge of cation = 0
A = charge of anion = 0
Now we have to determine the hybridization of the \(XeF_4\) molecule.
\(\frac{1}{2}[8+4-0+0]=6\)
Bond pair electrons = 4
Lone pair electrons = 2
The number of electrons are 6 that means the hybridization will be \(sp^3d^2\) and the electronic geometry of the molecule will be octahedral.
The molecular geometry will be square planar as two alternate positio ns will be occupied by lone pair of electrons so as to minimize the repulsion.
What does
the atomic mass tell you?
Answers
Answer:
how many protons and neutrons in its atoms
Explanation:
A scientist studies the growth of plants in a laboratory. Which experimental procedure would provide evidence that
plants need nitrogen to grow? (1 point)
Two plants are grown in pots of soil with different amounts of nitrogen. The plants have sufficient
water and sunlight. The scientist measures plant growth.
Seeds are planted in two identical pots of soil. Both pots are given adequate water and exposure to
sunlight. A plant grows in one pot. The scientist measures the nitrogen content of the soil
Identical plants are placed in two air-tight containers with sufficient exposure to sunlight. One
container has nitrogen gas, and the other has room air. The scientist measures plant growth
Seeds are planted in two terrariums. Both terrariums are given adequate water and exposure to
sunlight. A plant grows in one terrarium. The scientist measures the nitrogen content of the air.
Answers
The experimental procedure would provide evidence that plants need
nitrogen to grow is by
Identical plants are placed in two air-tight containers with sufficient exposure to sunlight. One container has nitrogen gas, and the other has room air. The scientist measures plant growthIt is necessary to use identical plants during the experiment as plants have
different rates of nitrogen absorption from the soil. It should also be done in
air-tight containers called terrariums to prevent loss or contamination of the
gases with other elements/compounds.
The identical plants containing nitrogen and room air should both be
measured for the growth difference between them.
Read more on https://brainly.com/question/972291
What changes sodium pellets to liquid
Answers
Answer:
when placed in water, a sodium pellet catches on fire as hydrogen gas is liberated and sodium hydroxide forms. chemical change = fire is a sign of chemical reaction.
Explanation:
When placed in water the sodium pellets catch the fire and liberate the hydrogen gas. On mixing with water solid sodium forms a colorless basic solution.
What are the properties of sodium?Sodium is a soft metal. It is a very reactive element with a low melting point. Sodium reacts very quickly with water, snow, and ice to produce sodium hydroxide and hydrogen. It is an alkali metal and the sixth most abundant metal on earth. It has a silvery white color.
It has a strong metallic luster. On reacting with oxygen it produces sodium oxide which on reacting with the water produces sodium hydroxide.
It is used to improve the structure of certain alloys and soaps. It is also used in the purification of metals. Sodium is also present in sodium chloride, an important compound found in the environment.
To learn more about sodium, refer to the link:
https://brainly.com/question/29327783
#SPJ2
What is the proton number for the particle missing from this beta decay?

Answers
The proton number for the particle missing from this beta decay is 4 and is therefore denoted as option C.
What is beta decay?This is referred to as a type of radioactive decay in which a proton is transformed into a neutron or vice versa inside the nucleus of the radioactive sample.
Proton number is also referred to as atomic number and in his scenario, the subscripts in the products and reactants have to be equal. This therefore means that 3 = x - 1
x = 3+1 = 4 which is therefore the reason why it was chosen as the correct choice.
Read more about Beta decay here https://brainly.com/question/12448836
#SPJ1
(c) 4 is the proton number for the particle missing from this beta decay
what is a proton?A stable subatomic particle with the symbol P, H+, or 1H+, a proton has an electric charge of +1 e. Its mass is 1,836 times greater than that of an electron and only slightly less than that of a neutron. Nucleons are the collective name for protons and neutrons, which have masses of roughly one atomic mass unit apiece.
what is an atomic mass?An atom's mass is its atomic mass. Although the kilogram is the SI measure of mass, the unified atomic mass unit, or dalton, is a common way to express atomic mass. An unbound carbon-12 atom in its ground state has a mass of 112 of a Da.
learn more proton
https://brainly.com/question/19332997
#SPJ1