Answers
Answer:
Explanation:
⁵⁹₂₆Fe --------- ⁰₋₁e + ⁵⁹₂₇Co
Co- 59 is known as Cobalt
its symbol is ⁵⁹₂₇Co
The name of the product is Cobalt-59 and the symbol of the nuclide is \(^{59}Co_{27}\).
Beta-decay:It is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide.
When the nuclide iron-59 undergoes beta decay it will lead to the formation of cobalt-59. The reaction can be represented as:
\(^{59}Fe_{26} --> ^0e_{-1} + ^{59}Co_{27}\\\)
Thus, The name of the product is Cobalt-59 and the symbol of the nuclide is \(^{59}Co_{27}\).
Find more information about Beta decay here:
brainly.com/question/1199820
Related Questions
What does physical science explore?
Answers
Answer:
The study of the inorganic world
Explanation:
That is, it does not study living things. (Those are studied in biological, or life, science.) The four main branches of physical science are astronomy, physics, chemistry, and the Earth sciences, which include meteorology and geology.
Answer:
physics is matter and energy. it explores mechanics, heat, light and other radiation, sound, electricity, magnetism, and the structure of atoms.
How much ice could be melted at 0°C if 5200 joules of heat were added?
Answers
Answer:
0.02kg
Explanation:
Given parameters:
Amount of heat = 5200J
Unknown:
Mass of ice that would be melted at 0°C = ?
Solution:
To solve this problem, use the expression below;
H = mL
H is the heat
m is the mass
L is the latent heat of fusion of ice = 3.33 x 10⁵ J/kg.
Insert the parameters and solve for m;
5200 = m x 3.3 x 10⁵
m = \(\frac{5200}{3.33 x 10^{5} }\) = 0.02kg
In the following combustion reaction you use 43.5 grams of C3H8. How many moles of H2O was produced?C3H8 + 5O2 → 3CO2 + 4H2O
Answers
Step 1 - Finding the stoichiometry of the reaction
The given reaction is:
\(C_3H_8+5O_2\to3CO_2+4H_2O\)The stoichiometry of the reaction can be found by reading the bigger numbers, those that come before the formulas of the substances:
1 mole of C3H8 react with 5 moles of O2 thus producing 3 moles of CO2 and 4 moles of H2O
Since the exercise is specifically asking us about the proportion between C3H8 and water, we can simplify the statement above to:
1 mole of C3H8 produces 4 moles of H2O
Step 2 - Converting the number of moles of C3H8 to grams
Note that the exercise is asking us about how much water would be produced if we used 43.5 grams of C3H8. For sake of simplicity, let's convert the number of moles of C3H8 to mass (grams).
Converting moles to grams is easy. We just have to multiply the number of moles by the molar mass of the substance (44 g/mol for C3H8):
\(C_3H_8\to1\times44=44\text{ g}\)We can now rewrite the statement in step 1 as:
44 g of C3H8 produce 4 moles of H2O
Step 3 - Finding how many moles of water would be produced
Now we just have to set a proportion:
\(\begin{gathered} 44\text{ g of C3H8 produce --- 4 moles of H2O} \\ 43.5\text{ g of C3H8 would produce -- x} \\ \\ x=\frac{4\times43.5}{44}=3.95\text{ moles of H2O} \end{gathered}\)Therefore, 3.95 moles of H2O would be produced in this reaction.
someone help with this will mark brainliest

Answers
Answer:
The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the spectrum, whereas an absorption spectrum has dark-coloured lines in the spectrum.l
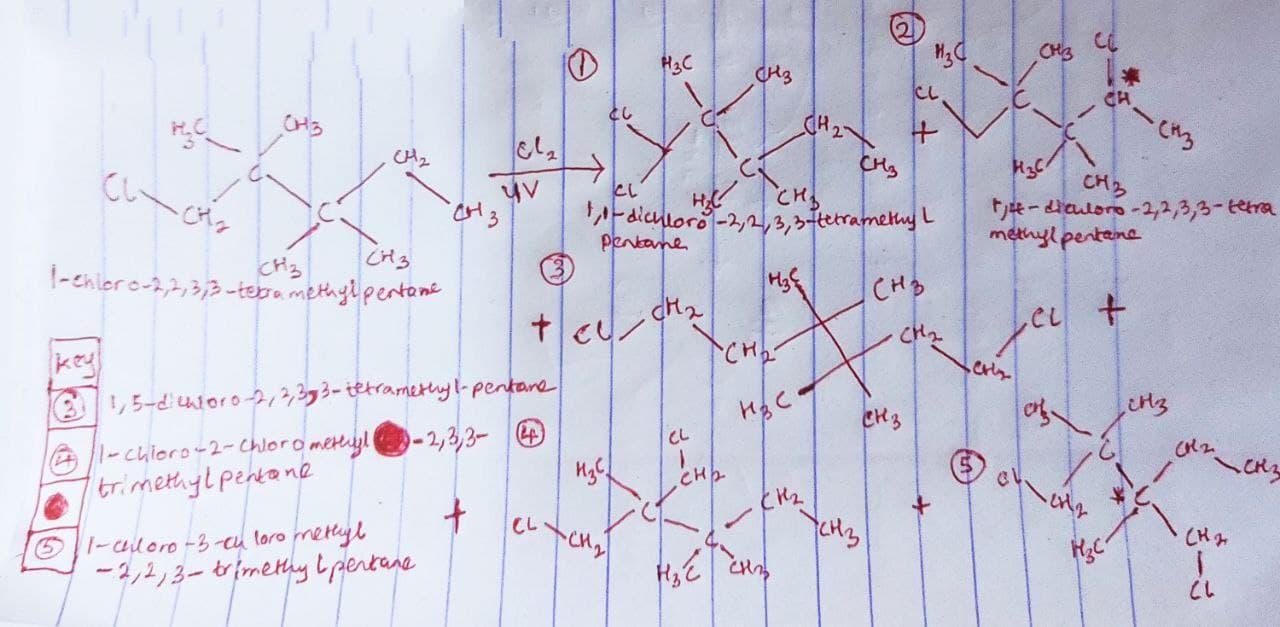
Draw bond-line formulas of all dichloro derivatives that might be formed when 1-chloro-2,2,3,3,-tetramethylpentane is allowed to react with Cl 2 under UV irradiation. For each structure, indicate, with an asterisk, any stereocenters that might be present. Predcit the percentage of each product using the relative reactivities 3 0 = 5.3, 2 0 = 3.6, 1 0 = 1
Answers
Answer:
Explanation:
This is a halogenation reaction i.e substitution or replacement of a single or more than a single hydrogen atom in the organic alkane compound with the halogen(here it is chlorine).
The chlorination of 1-chloro-2,2,3,3-tetramethylpentane under UV light resulted in the formation of five (5) dichloro derivatives which are shown in the image attached below.
Also, the compounds containing a stereocenter (i.e a location within the compound composing of various substituents in which the interchangeability of these substituents has the tendency of resulting into a stereoisomer) are indicated with an asterisk in the image below.
From the image below:
compound 1 ⇒ 1,1-dichloro-2,2,3,3-tetramethylpentane = 2° C
∴
The given relative reactivity rate for 2° = 3.6x
For compound 2 ⇒ 1,4-dichloro-2,2,3,3-tetramethylpentane = 2° = 3.6x
For compound 3 ⇒ 1,5-dichloro-2,2,3,3-tetramethylpentane = 1° = 1x
For compound 4 ⇒ 1-chloro-2-chloromethyl-2,3,3-trimethylpentane
= 1° = 1x
For compound 5 ⇒ 1-chloro-3-chloromethyl-2,2,3-trimethylpentane
= 1° = 1x
As such, we have:
2(3.6x) + 3(1x) = 100
7.2x + 3x = 100
10.2x = 100
x = 100/10.2
x = 9.803°
∴
For compound (1) = 3.6(9.803) = 35.3%
For compound (2) = 3.6(9.803) = 35.3%
For compound (3) = 1(9.803) = 9.803°%
For compound (4) = 1(9.803) = 9.803°%
For compound (5) = 1(9.803) = 9.803°%
if there are more products than reactants, does that mean there is an increase in the forward or backward reaction? And if there are more reactants that products, is there an increase in the forward or backward reaction?
Answers
Answer:
If there are more products than reactants, that means the reaction has shifted towards the left, which is the backward direction. If there are more reactants than products, that means the reaction has shifted towards the right, which is the forward direction.
The price of gold is $40.63/g. How many kg of gold would be worth $100?
Answers
Answer:
0.00246kg
Explanation:
1g = $40.63
$100 = $100/40.63 = 2.46g
2.46/1000 g = 0.00246kg
If 335 g of water at 65.5 °C loses 9750 J of heat,
what is the final temperature of the water? Liquid
water has a specific heat of 4.18 J/(g*°C).
Answers
Answer:
We can use the formula for heat lost by a substance to calculate the final temperature:
Q = m * c * ΔT
where Q is the heat lost, m is the mass of the substance, c is the specific heat capacity of the substance, and ΔT is the change in temperature.
In this case, we know the values of Q, m, and c, and we need to find ΔT. Rearranging the formula, we have:
ΔT = Q / (m * c)
Substituting the given values, we get:
ΔT = 9750 J / (335 g * 4.18 J/(g*°C)) ≈ 6.9 °C
Therefore, the final temperature of the water is:
65.5 °C - 6.9 °C ≈ 58.6 °C
So the final temperature of the water is approximately 58.6 °C.
The final temperature of the water is approximately 58.5°C.
To find the final temperature of the water, we first need to understand that the heat lost by the water is calculated using the formula q = mcΔT, where 'q' is the Heat Transfer, 'm' is the mass of the water, 'c' is the specific heat of the water, and 'ΔT' is the change in temperature.
First, rearrange the formula to find ΔT = q/(mc).
Then, insert the given values (q = -9750 J, m = 335 g, c = 4.18 J/g°C).
The negative sign denotes heat loss.
You will find ΔT is approximately -7°C.
This is the amount the temperature decreases.
Subtract ΔT from the initial temperature of the water (65.5°C - 7°C), to get the final temperature of approximately 58.5°C.
Learn more about Heat Transfer here:
https://brainly.com/question/34419089
#SPJ2
Koby wanted to do an experiment where he could observe a chemical reaction. He decided that he was going to combine baking soda with water and with vinegar and see whether either one created a chemical reaction.
Koby placed ½ teaspoon of baking soda into each of two identical glasses. He noticed the baking soda was lumpy, so he used a fork to break up the lumps. Why is it important to break up the lumps?
Answers
Koby needed to break the baking soda to provide a larger surface area for reaction thereby increasing the rate of reaction.
Rate of reaction?The term rate of reaction refers to how quickly or slowly a reaction proceeds. The surface area of solid reactants is an important factor that influences the rate of reactions when a solid reactant is involved.
Hence, Koby needed to break the baking soda to provide a larger surface area for reaction thereby increasing the rate of reaction.
Learn more about rate of reaction: https://brainly.com/question/8592296
Conductivity is an example of what type of property?
Answers
Answer:
A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity.
Explanation:
Conductivity is an example of a physical property. Physical properties are characteristics of a substance that can be observed or measured without changing the substance's chemical composition.
Conductivity specifically refers to a material's ability to conduct electricity or heat. Materials with high electrical conductivity allow electricity to flow easily through them, while materials with high thermal conductivity conduct heat efficiently.
Examples of conductive materials include metals like copper and aluminium, which are widely used in electrical wiring and heat transfer applications due to their excellent conductivity properties.
Learn more about Conductivity, here:
https://brainly.com/question/33823148
#SPJ6
How many moles of MgCl2 are present in 60.0 mL of 0.100 M MgCl2 solution
Answers
Taking into account the definition of molarity, the number of moles of MgCl₂ present in 60.0 mL of 0.100 M MgCl₂ solution is 0.006 moles.
Definition of molarityMolarity is a measure of the concentration of a solute in a solution and indicates the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by:
molarity= number of moles of solute÷ volume
Molarity is expressed in units moles/L.
Number of moles of MgCl₂In this case, you have:
Molarity= 0.100 Mnumber of moles of MgCl₂= ?volume= 60 mL= 0.06 L (being 1000 mL= 2 L)Replacing in the definition of molarity:
0.100 M=number of moles of MgCl₂÷ 0.06 L
Solving:
0.100 M × 0.06 L= number of moles of MgCl₂
0.006 moles= number of moles of MgCl₂
Finally, the number of moles of MgCl₂ is 0.006 moles.
Learn more about molarity:
brainly.com/question/9324116
brainly.com/question/10608366
brainly.com/question/7429224
#SPJ1
what is the change in mass of A in
60 minutes?
Mass of A (g)
12.4
10.4
9.1
7.7
6.2
Time
O
15
30
45
60
Answers
Answer:
To determine the change in mass of A over the given time period, we need to find the difference between the initial mass of A and the final mass of A.
From the given table, we can see that the initial mass of A at t = 0 (start time) is 12.4 g and the final mass of A at t = 60 minutes (end time) is 6.2 g.
Therefore, the change in mass of A over 60 minutes is:
Final mass of A - Initial mass of A
= 6.2 g - 12.4 g
= -6.2 g
The negative sign indicates that the mass of A decreased over time, which means that A underwent some kind of reaction or process that caused it to lose mass.
The change in mass of A over 60 minutes is -6.2 grams.
To determine the change in mass of A over 60 minutes, we need to compare the initial mass to the final mass.
From the given information, we can see that the mass of A decreases over time.
Let's calculate the change in mass.
Initial Mass of A: 12.4 g
Final Mass of A: 6.2 g
Change in Mass of A = Final Mass of A - Initial Mass of A
= 6.2 g - 12.4 g
= -6.2 g
The change in mass of A over 60 minutes is -6.2 grams.
Note that the negative sign indicates a decrease in mass.
For such more questions on mass
https://brainly.com/question/1838164
#SPJ8
The solubility in water of ionic compound X is measured and found to be 0.776g/mL at 15.°C. Calculate the volume of a saturated solution of X in water that would contain 34.0g of X at this temperature. Be sure your answer has the correct unit symbol and 3 significant digits.
Answers
Answer: The volume of a saturated solution of X in water that would contain 34.0g of X is 43.9 ml
Explanation:
Solubility is defined as the amount of solute dissolved per 100 ml of the solvent at a fixed temperatur.
Given : solubility of ionic compound X in water at \(15^0C\) = 0.776 g/ml
0.774 g of X is contained in = 1 ml
Thus 34.0 g g of X is contained in = \(\frac{1}{0.774}\times 34.0=43.9 ml\)
Thus the volume of a saturated solution of X in water that would contain 34.0g of X is 43.9 ml
Convert 100.6 Kelvin to degrees C.
°C = K - 273
[?] °C
Answers
Answer:
-172.6 °C
Explanation:
You want to know the Celsius equivalent of the temperature 100.6 K.
ConversionThe relation is ...
C = K - 273.15
C = 100.6 -273.15 = -172.55
The temperature is -172.55 °C, about -172.6 °C.
__
Additional comment
We have rounded to tenths, because that is precision of the temperature given. If you use 273 as the conversion constant, you will get -172.4.
Perform the following mathematical operation, and report the answer to the appropriate number of significant figures.
1204.2 + 4.72613 = [?]
The answer is not 1208.92613
Answers
1208.9213 not then what's the answer
Fet2 and Fe3 are
valences
isotopes
ions
Molecules
Answers
Answer:
Ions.
Explanation:
Hello!
In this case, since iron is a metal which has lots of uses in the design of metallic alloys and materials, when it is at its ground state we say it is just Fe; however, since it is a metal, it is very likely to lose electrons and therefore getting positively charged as +2 or +3, say:
\(Fe^{2+}\\\\Fe^{3+}\)
Thus, since they are positively charged, they are classified as cations, which are ions.
Best regards!
Can you help me with the state of matter part?

Answers
STEP-BY-STEP EXPLANATION
Sodium chloride (NaCl) exists in a solid state cause it possesses a high melting point. NaCl has a strong force attraction between sodium ion and chloride ion and this invole the transferring of electrons from sodium to chloride. Therefore, sodium chloride exists in the solid state.
\(Na^++Cl^-\text{ }\rightarrow\text{ NaCl}\)O Cr³+ because it loses electrons
O Na because it loses electrons
O Cr³+ because it gains electrons
O Na because it gains electrons
Cr³+ + 3Na
3Na+ + Cr
Answers
The symbol is shown as Cr³+ because it loses electrons. Option A
What is oxidation?When we talk about the process of oxidation, what is going on is the loss of electrons. Thus it is possible to say that ocidation is electron loss. The electrons that are lost would lead to the formation of a specie that has a positive ion.
The magnitude of the positive charge that we see in the compound is based on the number of electrons that it has lost in the process of the oxidation of the compound. There are three electrons that have been lost for chromium as shown.
Learn more about oxidation:https://brainly.com/question/9496279
#SPJ1
place the following in order of increasing bond length. c-fc-sc-cl a) c-s < c-cl < c-f b) c-cl < c-f < c-s c) c-f < c-s < c-cl d) c-f < c-cl < c-s e) c-s < c-f < c-cl
Answers
The order of increasing bond length is d) c-f < c-cl < c-s
The bond length of a covalent bond is determined by the size of the atoms involved and the strength of the bond. The size of the atoms involved in the c-f, c-s, and c-cl bonds are carbon, fluorine, sulfur, and chlorine, respectively. Carbon and fluorine are smaller atoms, while sulfur and chlorine are larger atoms. Therefore, the c-f bond will be the shortest bond.
The strength of the covalent bond is determined by the electronegativity of the atoms involved. Fluorine has the highest electronegativity of all the elements, followed by chlorine and then sulfur. Therefore, the c-f bond will be the strongest bond. The c-cl bond will be the second strongest bond, and the c-s bond will be the weakest bond.
Given these facts, the order of increasing bond length is c-f < c-cl < c-s. The c-f bond will be the shortest and strongest bond, followed by the c-cl bond and then the c-s bond.
For more questions like Bond length click the link below:
https://brainly.com/question/5129537
#SPJ4
At a certain temperature it is found that 1.83 moles of H2, 2.33 moles of 02 and 3.95 moles of H2O are in equilibrium in a 8.1 L container according to the reaction below. What is the equilibrium constant?
2 H2 (g) + 02 (g) = 2 H20 (g)
Keep extra significant figures during the calculation and round your answer to 1 decimal place.
Answers
0.6 is the equilibrium constant for the given reaction.
To calculate the equilibrium constant (K) for the given reaction, we need to use the molar concentrations of the reactants and products at equilibrium. The equilibrium constant expression is given by:
\(K= [H_{2}O]^{2} / ([H_{2}^{2} * [O_{2}])\)
Given the moles of H2, O2, and H2O in the 8.1 L container, we can convert them to molar concentrations by dividing the number of moles by the volume:
[H2] = 1.83 moles / 8.1 L
[O2] = 2.33 moles / 8.1 L
[H2O] = 3.95 moles / 8.1 L
Substituting these values into the equilibrium constant expression, we have:
K = \((3.95 / 8.1)^{2}\) / (\((1.83 / 8.1)^{2}\) * (2.33 / 8.1))
Evaluating this expression and rounding to one decimal place, we find the equilibrium constant to be:
K ≈ 0.6
Therefore, the equilibrium constant for the given reaction is approximately 0.6.
Know more about equilibrium constant here:
https://brainly.com/question/3159758
#SPJ8
Calculate ph of 1,0 mol/l of solution NH4Cl
Answers
Answer:
4.74.
Explanation:
The equilibrium equation for the hydrolysis of NH4+ is:
NH4+ + H2O ⇌ NH4OH + H+
The Cl- ion is a strong base and will not undergo hydrolysis in water. Therefore, the Cl- ion will not affect the pH of the solution.
Use the equilibrium constant for the hydrolysis of NH4+ (Kb) to calculate the pH of the solution.
The expression for the equilibrium constant for the hydrolysis of NH4+ is:
Kb = [NH4OH][H+] / [NH4+]
Given that the concentration of NH4Cl is 1.0 mol/L, the concentration of NH4+ ions is also 1.0 mol/L.
The concentration of hydroxide ions (OH-) can be calculated from the Kb expression:
Kb = [NH4OH][H+] / [NH4+]
[OH-] = Kb * [NH4+]
Knowing that the Kb for NH4+ = 1.810^-5
[OH-] = 1.810^-5 * 1.0 = 1.8*10^-5 M
The pH of the solution is the negative logarithm of the hydroxide ion concentration:
pH = -log([OH-])
pH = -log(1.8*10^-5)
pH = 4.74
Therefore, the pH of 1.0 mol/L solution of NH4Cl is 4.74.
Which equation correctly converts degrees Celsius to kelvins?
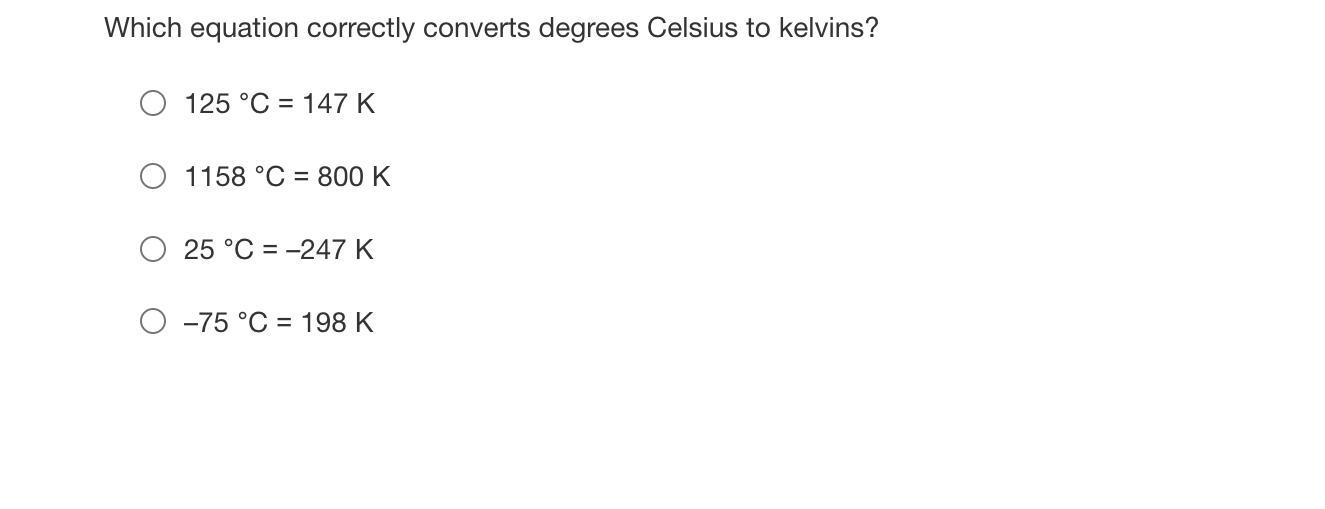
Answers
Answer:
D
Explanation:
To convert from celcius to kelvin add 273 to the celcius number. D has -75 celcius so if you add -75 to 273 you get 198 K
The correct equation that converts degree celsius to kelvin is -75°C = 198K
To convert from celcius to kelvin we have to add 273 to the celcius number.
i.e degree celsius + 273= kelvin
option D has -75 celcius so if you add -75 to 273 we get 198 K.
A kelvin is the same size as a Celsius degree.
Absolute zero is 0 K and the freezing point of water is 273.15 K.
To get the freezing point of water to be 0 °C, we must subtract 273.15 from the Kelvin reading (see Figure).
C = K – 273.15
C = 273.15 – 273.15
C = 0 °C
To get absolute zero on the Celsius scale, we repeat the process.
C = 0 – 273.15
C = –273.15 °C
To know more about kelvin here
https://brainly.com/question/11631512
#SPJ2
What does cellular respiration do?
Break down sugar and release energy for an
organism to use
Create sugar filled with energy
Deter predators
Form glucose from hydrogen, oxygen, and carbon
:D
help asap
Answers
4.If 15.00 mL of 3.00 M potassium iodide is needed to reach the equivalence point with 10.00 mL of lead (Il) nitrate, determine the molarity of the lead (Il) nitrate solution
Answers
Answer:
2.25 M
Explanation:
The reaction that takes place is:
2KI + Pb(NO₂)₃ → PbI₂ + 2KNO₃First we calculate how many potassium iodide moles reacted, using the given volume and concentration:
15.00 mL * 3.00 M = 45 mmol KIThen we convert 45 mmoles of KI into mmoles of Pb(NO₂)₃, using the stoichiometric coefficients of the balanced reaction:
45 mmol KI * \(\frac{1mmolPb(NO_3)_2}{2mmolKI}\) = 22.5 mmol Pb(NO₂)₃Finally we calculate the molarity of the Pb(NO₂)₃ solution, using the calculated number of moles and given volume:
22.5 mmol Pb(NO₂)₃ / 10.00 mL = 2.25 MWhy does the solubility of alkaline earth metal hydroxides in water increase down the group?
Answers
Answer:
The solubility of alkaline earth metal hydroxides in water increases down the group because the size of the metal cation increases as you move down the group. This increase in size results in a decrease in the cation's charge density, which makes it less able to attract and hold onto hydroxide ions. As a result, the hydroxides become more soluble in water as you move down the group. Additionally, the lattice energies of the hydroxides decrease down the group, making it easier to break apart the crystal lattice structure and dissolve the hydroxides in water.
In the following net ionic equation, identify each reactant as either a Bronsted-Lowry acid or a Bronsted-Lowry base.
HF-(aq) + H2O(l) rightarrow F-(aq) + H3O(aq)
B-L_____ B-L_____
The formula of the reactant that acts as a proton donor is_____.
The formula of the reactant that acts as a proton acceptor is_______.
Answers
Answer:
Bronsted lowry base = Proton acceptor = H2O
Bronsted lowry acid = Proton donor = HF-
Explanation:
The equation is given as;
HF-(aq) + H2O(l) --> F-(aq) + H3O(aq)
A bronsted lowry base is any specie that can accept hydrogen ion (proton) from another molecule.
Basically a bronsted lowry base is a proton acceptor while a bronsted lowry acid is a proton donor.
In the reaction above, upon comparing both the reactants and products;
Bronsted lowry base = Proton acceptor = H2O
Bronsted lowry acid = Proton donor = HF-
The layer of rock that forms Earth's outer
surface
Answers
Answer:
Crust
Explanation:
Answer:
The crust
Explanation:
What is the chemical formula for copper(II) sulfate?
O CuSO4
O Cu₂SO4
O CuS
O Cu₂S
Answers
The chemical formula for copper(II) sulfate is CuSO4.
What is copper(II) sulfate?Copper(II) sulfate is a chemical compound that is made up of copper, sulfur, and oxygen. It has the chemical formula CuSO4 and is commonly referred to as "blue vitriol" or "bluestone." Copper(II) sulfate can be prepared by reacting copper oxide or copper metal with sulfuric acid. It is a blue-colored crystalline solid that dissolves easily in water.
Copper(II) sulfate has many uses in industry and can be used as a fungicide, herbicide, pesticide, and in the manufacture of other chemicals. It is also commonly used in schools and laboratories as a reagent in chemical reactions and experiments.
Learn about copper(II) sulfate here https://brainly.com/question/30459517
#SPJ1
Which two particles are found in the nucleus of an atom?
neutrons and electrons
protons and electrons
protons and neutrons
neutrons and atoms
Answers
Answer:
C.) protons and neutrons
Explanation:
Most atoms contain proton(s), neutron(s), and electron(s). Within the nucleus of an atom, there are protons and neutrons. Electrons are located outside of the nucleus.
The solubility product for an insoluble salt with the formula MX4 is written as ________, where s is the molar solubility. Select one: a. Ksp
Answers
Answer:
Ksp = 256(s)⁵
Explanation:
The given insoluble salt dissolves as:
\(MX4 <-----> M4+ \ \ \ \ \ \ + \ \ \ \ \ \ \ 4X -\)
s 4s
The solubility product is the product of individual solubilities of the cation and anion of a salt.
\(Ksp = [M4+][X^-]^4\)
\(Ksp = [s][4s]^4\)
Ksp = 256(s)⁵