when treated with sodium borohydride, d-glucose is converted into an alditol.T/F
Answers
True, when treated with sodium borohydride, D-glucose undergoes reduction and is converted into D-glucitol or sorbitol, which is an alditol.
The reaction of D-glucose with sodium borohydride (NaBH₄) forms an alditol.
Sodium borohydride is a reducing agent that reduces the aldehyde group (-CHO) present in D-glucose to form an alcohol group (-OH).
Here, the reduction process goes as;
R-CHO + NaBH₄ + mild acid (for workup) → R-CH2-OH
The addition of one H-atom at carbon and the other at oxygen reduces the aldehyde to alcohol.
Therefore, this results in the formation of an alditol, specifically D-glucitol (also known as sorbitol).
To know something about d-glucose, click below.
https://brainly.com/question/17179052
#SPJ11
Related Questions
what is the purpose of washing the precipitate with hot water in step 3(a) of the procedure? be as specific as possible in your answer
Answers
In the procedure, step 3(a) states that washing the precipitate is necessary. The reason for washing the precipitate with hot water is that it removes any remaining impurities and unreacted chemicals.
Washing the precipitate helps to purify it and remove unwanted particles. Hot water is used because it can dissolve impurities and wash them away more effectively than cold water. Additionally, the hot water can increase the rate of precipitation, making the process faster. If the precipitate is not washed properly, it can have a negative effect on the final product. The washing process ensures that the precipitate is pure and ready for further use. Overall, washing the precipitate is a crucial step in the procedure to ensure the purity and quality of the final product.
To know more about impurities visit:
https://brainly.com/question/32369276
#SPJ11
Help pls , question is in picture

Answers
Explanation:
IM PRETTY SURE IT IS D !! IF ITS WRONG IM SORRY THAT WHAT
I GOT
A piece of cork has a volume of 35.5 milliliters, ml. what is the mass of the piece of cork in grams, g?
Answers
The mass of the piece of cork is determined by its density, which can vary depending on the specific type of cork.
To calculate the mass of the piece of cork, we need to know its density. Density is defined as the mass per unit volume of a substance. However, the question only provides the volume of the cork, which is 35.5 milliliters (ml). Without the density, we cannot directly determine the mass.
To find the mass, we need to multiply the volume by the density of the cork. Density is typically expressed in grams per milliliter (g/ml) or grams per cubic centimeter (g/cm³). Once we have the density, we can use the formula:
Mass (g) = Volume (ml) x Density (g/ml)
Without information about the density of the cork, we cannot accurately calculate its mass. Therefore, we need additional data or specifications to proceed with the calculation.
Learn more about mass
brainly.com/question/11954533
#SPJ11
For an ecosystem to be healthy, it has to have lots of different kinds of plants and animals.
Answers
Answer:
hey mate here is your answer
Biodiversity is a term used to describe the enormous variety of life on Earth. It can be used more specifically to refer to all of the species in one region or ecosystem. Biodiversity refers to every living thing, including plants, bacteria, animals, and humans.
please mark me as a brainliest
How many molecules are equal to 10.35 g of pyridine?
Answers
Answer:
0.78× 10 ²³ molecules
Explanation:
Given data:
Mass of pyridine = 10.35 g
Number of molecules = ?
Solution:
Number of moles of pyridine:
Number of moles = mass/molar mass
Number of moles = 10.35 g/ 79.1 g/mol
Number of moles = 0.13 mol
Number of molecules;
1 mole contain 6.022× 10 ²³ molecules
0.13 mol × 6.022× 10 ²³ molecules / 1mol
0.78× 10 ²³ molecules
As a forensic expert, Jane needs to handle many biological evidence samples simultaneously. What is the basic precaution that she should take
while doing so?
OA
air dry all the samples
B.
use a new pair of gloves for each piece of evidence
malaman
store samples in humid conditions
Ос.
OD.
use plastic bag for storage
Answers
Answer:
B. use a new pair of gloves for each piece of evidence
Explanation:
When it comes to the basic precautions of handling biological evidence samples, one has to prevent cross contamination. This refers to the transfer of DNA from one evidence to another evidence; thus, it is important for Jane to use a new pair of gloves for each piece of evidence in order to prevent such occurrence.
Wearing of gloves will also prevent Jane's DNA from being implanted into the sample, and it will keep her safe from contracting blood-borne pathogens like those in the saliva, blood or semen.
When hydrochloric acid is passed through a saturated solution of common salt, a precipitate of _______ will form.
Answers
When hydrochloric acid (HCl) is passed through a saturated solution of common salt (NaCl), a precipitate of sodium chloride (NaCl) will form.
This reaction occurs due to a chemical reaction between the hydrochloric acid and the sodium chloride in the solution. Hydrochloric acid is a strong acid that dissociates into hydrogen ions (H+) and chloride ions (Cl-) in water. When it is added to a saturated solution of common salt, which already contains dissolved sodium ions (Na+) and chloride ions (Cl-), a precipitation reaction takes place. The hydrogen ions from the acid combine with the chloride ions from the salt solution to form hydrochloric acid molecules (HCl) again.
The remaining sodium ions and chloride ions in the solution are now in excess, causing the solubility of sodium chloride to be exceeded. As a result, solid sodium chloride particles begin to form and settle as a precipitate in the solution. This precipitation reaction is a common occurrence when mixing hydrochloric acid with a saturated solution of common salt.
The formed precipitate of sodium chloride appears as fine crystals or solid particles, depending on the concentration of the salt solution and the amount of hydrochloric acid added. These particles can be separated from the solution through filtration or other appropriate techniques.
In summary, when hydrochloric acid is passed through a saturated solution of common salt, a precipitate of sodium chloride will form due to the chemical reaction between the acid and the salt solution.
for more such questions on precipitate
https://brainly.com/question/30763500
#SPJ11
Joy mixed baking soda and vinegar for her volcano model. She saw lots of bubbling and fizzing. What can she infer happened
A chemical change
A change in state liquid to solid
Matter was created
A physical change
Answers
Answer:
a chemical change
Explanation:
bubbles formed
Answer:
Explanation:
✔︎A chemical change
✖︎wich is D
would be ur answer
Explanation:
❐A chemical change happens when one chemical substance is transformed into one or more different substances, such as when iron becomes rust. Chemical changes occur through the process of chemical reactions, and the resulting substances have different properties because their atoms and molecules are arranged differently.
CAN I PLZ GET MARK AS THE BRAINLIEST? ")
At room temperature Bromine is a liquid and lodine is a solid, what can be inferred
about the intermolecular forces of these two elements?
Answers
Answer:
Explanation:
The intermolecular forces are stronger in Iodine, meaning that its molecules are stronger and can hold that solid structure at room temperature, where Bromine cannot due to its IMF's being weaker.
which period of the periodic table is completely made up of elements with no known stable ions?
Answers
Answer:
I think the answer is period 7
Explanation:
The period in periodic table, in which no stable ions of elements exists is 7th period. They contains actinides.
What are actinides?Actinides are 7th period elements classified to F block of periodic table. A periodic table contains vertical columns called groups and horizontal rows called periods. Each elements are classified into suitable groups based on their similarity in properties with other group elements.
There are four blocks in periodic table which are s, p, d and f block. S block elements are metals and d block contains transition metals and p block have non metals including gases and metalloids.
Actinides are f- block metals. All the elements in the period are radioactive. That's why they don't have a stable isotope or ion. They continuously undergo radioactive decay by the emission of charged particles.
Hence, the period containing unstable elements is 7th one.
To find more about actinides, refer the link below:
https://brainly.com/question/5188867
#SPJ2
The trail making tests evaluates several cognitive skills, including a. mood, attention, and sequencing. b. attention, intelligence, and thought processing. c. attention, sequencing, and thought processing. d. intelligence, sequencing, and thought processing.
Answers
The trail making tests evaluate several cognitive skills, including attention, sequencing, and thought processing. The correct option is C.
The trail making tests are considered a measure of executive functioning, which encompasses a range of cognitive processes involved in planning, organizing, and regulating behavior. Specifically, these tests evaluate attention, sequencing, and thought processing. Attention refers to the ability to sustain and focus one's attention on a task, while sequencing refers to the ability to organize information in a logical sequence.
The trail making test is a neuropsychological assessment that measures cognitive functions such as attention, sequencing, and thought processing. It does not directly assess mood, intelligence, or overall intelligence. Instead, it focuses on an individual's ability to connect a sequence of numbers and letters, requiring them to switch between different cognitive processes.
To know more about attention visit:-
https://brainly.com/question/29739730
#SPJ11
Silver nitrate also forms a precipitate with nai. What would this precipitate be?.
Answers
When silver nitrate (AgNO₃) reacts with sodium iodide (NaI), a precipitate forms. The precipitate that is produced in this reaction is silver iodide (AgI).
The reaction can be represented by the following balanced chemical equation:
2AgNO₃ + 2NaI → 2AgI + 2NaNO₃
In this equation, two moles of silver nitrate react with two moles of sodium iodide to yield two moles of silver iodide and two moles of sodium nitrate.
Silver iodide is a yellow, crystalline solid that is insoluble in water. The formation of a precipitate occurs because silver iodide has low solubility, meaning it does not readily dissolve in water. Instead, it separates out of the solution as a solid, forming a visible precipitate.
The reaction between silver nitrate and sodium iodide is a double displacement reaction. The silver ions (Ag+) from silver nitrate combine with the iodide ions (I-) from sodium iodide to form solid silver iodide (AgI). The sodium and nitrate ions remain in solution as sodium nitrate (NaNO3).
Precipitation reactions are commonly used in chemical analysis to identify the presence of specific ions in a solution. In this case, the formation of the yellow precipitate of silver iodide confirms the presence of iodide ions in the solution.
To know more about double displacement reaction, refer to the link below:
https://brainly.com/question/13854110#
#SPJ11
Mrs. Keep burns a walnut under a beaker of water. The beaker contains 100 g of water which warms from 25oC to 30oC. Assuming that all the heat from the burning walnut goes into the water and none of the heat is lost to the air or the beaker, how many calories are in the walnut?
a 2100 calories
b 10,500 calories
c not enough information is given
d 500 calories
Answers
The amount of heat gained by the water is 500 calories. Thus, option D is correct.
Given:
Mass of water (m) = 100 g
Change in temperature (ΔT) = 30°C - 25°C = 5°C
The specific heat capacity of water (c) is approximately 1 calorie/gram°C.
Now, the amount of heat gained by the water,
Q = mcΔT
Where:
Q is the heat gained or lost by the substance
m is the mass of the substance
c is the specific heat capacity of the substance
ΔT is the change in temperature
Plugging in the values into the formula:
Q = 100 × 1 × 5
Q = 500 calories
Therefore, the amount of heat gained by the water is 500 calories.
Learn more about heat, here:
https://brainly.com/question/31608647
#SPJ1
If you use 25 grams of Lead (II) nitrate and 30 grams of sodium iodide, which one is the limiting reactant? and How many grams of sodium nitrate is formed?
Answers
Answer:
Limiting reactant: lead(II) nitrate (Pb(NO3)2).
Mass of sodium nitrate (NaNO3) = 12.92 g.
Explanation:
What is given?
Mass of lead (II) nitrate (Pb(NO3)2) = 25 g.
Mass of sodium iodide (NaI) = 30 g.
Molar mass of Pb(NO3)2 = 331 g/mol.
Molar mass of NaI = 150 g/mol.
Molar mass of sodium nitrate (NaNO3) = 85 g/mol.
Step-by-step solution:
First, let's state the balanced chemical equation. Lead (II) nitrate (Pb(NO3)2) reacts with sodium iodide (NaI) in a double-replacement reaction to produce sodium nitrate (NaNO3), and PbI2:
\(Pb(NO_3)_2+2NaI\rightarrow2NaNO_3+PbI_2.\)Now, let's calculate the number of moles of each reactant using its molar mass. The conversion from grams to moles for Pb(NO3)2 will look like this:
\(25\text{ g Pb\lparen NO}_3)_2\cdot\frac{1\text{ mol Pb\lparen NO}_3)_2}{331\text{ g Pb\lparen NO}_3)_2}=0.076\text{ moles Pb\lparen NO}_3)_2.\)And for NaI:
\(30\text{ g NaI}\cdot\frac{1\text{ mol NaI}}{150\text{ g NaI}}=0.20\text{ moles NaI.}\)The next step is to see how many moles of NaNO3 are being produced. We're going to need the chemical equation: let's start with Pb(NO3)2. 1 mol of Pb(NO3)2 reacted produces 2 moles of NaNO3, so we will obtain:
\(0.076\text{ mol Pb\lparen NO}_3)_2\cdot\frac{2\text{ moles NaNO}_3}{1\text{ mol Pb\lparen NO}_3)_2}=0.152\text{ moles NaNO}_3.\)And now, let's see that 2 moles of NaI reacted produce 2 moles of NaNO3, so the molar ratio between these compounds is 1:1, which means that 0.20 moles of NaI reacted will produce 0.20 moles of NaNO3 too:
\(0.20\text{ moles NaI}\cdot\frac{2\text{ moles NaNO}_3}{2\text{ moles NaI}}=0.20\text{ moles NaNO}_3.\)Based on these calculations, you can note that the limiting reactant would be Pb(NO3)2 because this compound imposes the limit because is being consumed first, it is producing the maximum amount of NaNO3 that we can produce in this reaction.
The final step is to calculate the mass of NaNO3 that is being produced. Remember as Pb(NO3)2 is the limiting reactant and it produces 0.152 moles of NaNO3, we use this data to find the mass of NaNO3 using its given molar mass too, like this:
\(0.152\text{ moles NaNO}_3\cdot\frac{85\text{ g NaNO}_3}{1\text{ mol NaNO}_3}=12.92\text{ g NaNO}_3.\)The answer is that the limiting reactant is lead (II) nitrate (Pb(NO3)2) and we're producing 12.92 g of sodium nitrate (NaNO3).
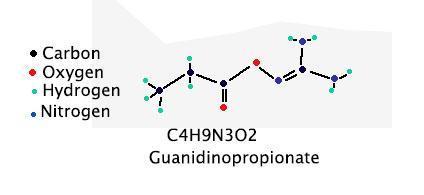
does someone know how to draw a structural formula of H1O2N3C4 please
Answers
Answer:
This could be guanidinopropionate. A drawing of the structure is attached.
Explanation:
There are other possible structures with this formula. The one shown in the attached diagram is one possibility. It is named, unfortunately, guanidinopropionate.
give the reagents/conditions used for the following reaction. group of answer choices conc. h2so4, heat naome naoh h2o
Answers
The reagents/conditions used for the following reaction are: Conc. \(H_2SO_4\), heat: This indicates the use of concentrated sulfuric acid (\(H_2SO_4\)) and heat as the reaction conditions. The heat is typically applied to initiate or speed up the reaction.
NaOH: Sodium hydroxide (NaOH) is a strong base that can be used in various reactions, such as neutralizations or hydrolysis reactions. It is often used in aqueous solutions.
\(H_2O\): Water (\(H_2O\)) is commonly present as a solvent or a reactant in many chemical reactions. It is necessary for the dissolution of certain compounds and can participate in hydrolysis reactions.
Please note that without specific context or reaction equation, it is challenging to determine the exact purpose or outcome of the reaction involving these reagents /conditions. The specific reaction and reactants involved would provide more information for a comprehensive understanding of the reaction.
To know more about sulfuric acid refer here
https://brainly.com/question/30039513#
#SPJ11
a substance x decomposes in a second-order reaction. a solution that is initially 1.00 m in x requires 0.50 h for its concentration to decrease to 0.50 m. how much time will it take for a solution of x to decrease in concentration from 1.00 m to 0.25 m? (a) 0.50 h (b) 1.0 h (c) 1.5 h (d) 2.0 h
Answers
A second-order reaction results in the breakdown of substance X. It takes 0.50 0.025 h for just a solution with an initial concentration of 1.00 0.5 M in X to degrade to 0.025 M 0.50M.
Reaction is what?
reaction definition 1 a: the reactionary act, process, or instance b: a power, influence, or activity that is opposed or opposed to especially: propensity for an old, typically obsolete political, social, or policy
What does a response time test entail?
regarding the test. This is a straightforward tool for calculating reaction times. According to the information gathered so far, the response time was 215 milliseconds on average (median). The latency of the computer and screen has an impact on this test in addition to assessing your reaction time.
To know more about reaction visit:
https://brainly.com/question/28984750
#SPJ4
could you unambiguously distinguish between borneol and isoborneol using 13c nmr spectroscopy? if not, why not?
Answers
Indeed, 13C NMR spectroscopy can be used to differentiate between borneol and isoborneol. Because the chemical structures of borneol and isoborneol differ.
A potent analytical method for examining the carbon nuclei in molecules is 13C NMR spectroscopy. It is based on the observation of the resonance frequencies of various carbon atoms within a molecule through the interaction of carbon-13 nuclei with an external magnetic field. The identification, quantification, and structural clarification of a wide variety of organic compounds are made possible by the information that 13C NMR spectra provide about the chemical environment and connectivity of carbon atoms in molecules. It has become a vital instrument for the characterisation of complex mixtures, natural products, and synthesised substances and is frequently utilised in disciplines including chemistry, biochemistry, and pharmacology.
Learn more about 13C NMR spectroscopy here:
https://brainly.com/question/13130554
#SPJ4
Distinguish between pure and applied science.
Answers
Answer:
pure science is the study of nature and its environment while applied science is the acquisition of scientific knowledge from pure science to solve practical problems
An atom has 17 protons, 18 neutrons and 17 electrons. What is the charge of its electron cloud?
Answers
Answer:
Neutral. There are equal amounts of protons or positively charged parts and electrons or negatively charged parts. The neutrons have no net charge which means this atom of chlorine is neutral.
Explanation:
Number of moles in 31.99g O2
And 176.8g NaCl
Answers
#O_2
\(\\ \sf\longmapsto No\:of\;moles=\dfrac{Given\:mass}{Molar\:mass}\)
\(\\ \sf\longmapsto No\:of\:moles=\dfrac{31.99}{32}\)
\(\\ \sf\longmapsto No\:of\:moles\approx 1\)
#NaCl
\(\\ \sf\longmapsto No\:of\:moles=\dfrac{176.8}{58.5}\)
\(\\ \sf\longmapsto No\;of\:moles=3mol\)
A reaction occurs that results in more products than reactants. What type of reaction is
'this?
Answers
Answer:
Endothermic reaction
Explanation:
Endothermic means that energy is absorbed leading to more product.
The reaction in which the products can return back to reactants is called reversible reaction. The reaction where more products are formed is called endothermic reaction where the products are more stable.
What is endothermic reaction?An endothermic reaction is a reaction where, the reactants absorbs heat energy and to produce new products. The term for heat absorption is called endothermic. Whereas the reaction in which heat is evolved is called an exothermic reaction.
In endothermic reaction, reactants gains thermal energy to overcome the energy barrier for the reaction. Therefore the activation energy for products will also be lower indicates their stability.
The enthalpy change that the change in heat absorbed for the endothermic reaction will be positive that is ΔHrxn is +ve. Endothermic phase change involves an increase in entropy. Hence, the reaction that result in more products than reactants is endothermic.
To find more on endothermic reactions, refer here:
https://brainly.com/question/23184814
#SPJ2
Name a non-metal used to make electrode in the cell.
Answers
Answer:
graphite
Explanation:
graphite is also good conductor of electricity
A cell is a system of parts that work together to carry out basic life processes. An animal is also a system of parts that work together to carry out basic life processes. Match each organ or organ system with the cell part that has a similar function.

Answers
Cell wall would be the skin
Cell membrane is the skeletal system
Mitochondria is the digestive system
I hope I’m right
Answer: the cell wall is the skeletal system
The nucleus is the brain
The mitochondria is the digestive system
The cell membrane is the skin
Explanation:
What is one Scientific Law or Scientific Theory that affects our life?
Answers
Answer:
Does the Big Bang theory count?
what is the molarity of 25 ml an acetic acid solution that contains 0.024 moles? report your answer to three decimal places.
Answers
The molarity of the acetic acid solution is 0.96 M, rounded to three decimal places.
It is calculated by dividing the number of moles of acetic acid by the volume of the solution in liters.
First, convert the volume from milliliters to liters by dividing by 1000:
25 ml = 25/1000 L = 0.025 L
Then, divide the number of moles (0.024) by the volume in liters (0.025):
Molarity = 0.024 moles / 0.025 L = 0.96 M
The density of acetic acid is 1.05 g/ml, and its molecular weight is 60 g/mol. Acetic acid CH3COOH (C2H4O2) has a molecular weight of 60 grams. One litre of water and one mole of acetic acid combine to form a molar solution.
To know more about the acetic acid refer here :
https://brainly.com/question/15202177#
#SPJ11
The radioactive isotope used in the dating of fossils thought to be less than 40,000 years old is ________________.
Fill in the blank.
Answers
Answer:
The answer is Radiocarbon
14. The atoms of element X contains nineteen electrons. With which of the following elements will the chemistry of Z be similar? a Aluminum b) Bromine c) Lithium d) Magnesium
Answers
First of all, Z is unknown. I hope it is a mistake.
Now, it is given that the element X has nineteen electrons. This proves that X is actually Potassium.
As per the periodic table, both Potassium and Lithium belongs to group 1 as their valency is 1 because of the presence of only one electron in the outermost shell of electrons i.e., they lose an electron during a chemical reaction to form a stable compound. Furthermore, both are metallic.
Magnesium belongs to group 2 and hence its valency is two, which is different from potassium though it is metallic. Similiarly, bromine belongs to group 17 and gains one electron during a reaction in contrast to potassium.
( No internal links available for reference. For clarification, check the periodic table).
calculate the food coloring concentrations in a standard if 3 ml of .892%(v/v) stock solution was transferred to a 100ml volumetric flask and diluted to volume
Answers
We can use the following formula to determine the amount of food colouring present in a standard solution: C1V1 = C2V2. As a result, the final standard solution's food colouring concentration is 0.02676% (v/v).
A homogeneous mixture of two or more substances, in which the solute is uniformly dispersed in the solvent, is referred to as a solution. Solutions exist in a variety of forms, including gases, liquids, and solids. They are crucial in a number of disciplines, such as chemistry, medicine, and technology. To create a solution, one must correctly measure the amounts of the solute and solvent and then completely combine them to create a homogeneous mixture. A solution's concentration can be stated in a number of ways, including molarity, molality, and percent by mass or volume. Solutions can differ from their component elements and compounds in terms of their boiling and melting points, as well as their appearance, flavour, and odour.
Learn more about Solution here:
https://brainly.com/question/2709960
#SPJ4
How is a TV like our brain?
Answers
Answer:
Explanation:
Because your brain and the TV are programed to do certain things. As if you're going back into a memory, and you're simply rewatching a season of Beyblade: Metal Fusion on the TV.