Answers
———
sulphur has atomic number 16, which signifies that it has 16 electron and 16 protons and 16 neutron
Related Questions
During the reaction, the energy is N2+3H2 2H3 is
Answers
Answer:
The reaction N2 + 3H2 → 2NH3 is an exothermic reaction, which means that it releases heat. The amount of energy released is called the enthalpy of reaction, and it is typically measured in kilojoules per mole (kJ/mol). The enthalpy of reaction for the Haber-Bosch process, which is the industrial process used to produce ammonia, is -46.2 kJ/mol. This means that for every mole of ammonia produced, 46.2 kJ of heat is released.
The energy released during the Haber-Bosch process is used to heat the reactants and to drive the reaction forward. The reaction is exothermic because the bonds in the products (NH3) are stronger than the bonds in the reactants (N2 and H2). When the reactants are combined, the energy released when the bonds in the products form is greater than the energy required to break the bonds in the reactants. This excess energy is released as heat.
The Haber-Bosch process is a very important industrial process, and it is used to produce ammonia on a large scale. Ammonia is used in a variety of products, including fertilizers, explosives, and plastics. The exothermic nature of the Haber-Bosch process makes it a very efficient way to produce ammonia.
Explanation:
What would
happen to the
vultures if the
gazelle
decreased?
Answers
Answer: If the gazelle population starts to decrease this could lead to some vultures not having enough food to eat
Explanation: This explains the obvious outcome of what would happen.
Hope you do well with what your doing :)
Mark as Brainliest please :)
Arrange these acids according to their expected pKą values. (Highest to lowest)
CH₂CH₂COOH
CI2CHCOOH
CICH₂COOH
CICH₂CH₂COOH
Answers
what is the name of this structure here please and thanks

Answers
Answer:
look at the file that the other person sent
Draw the skeletal structure of a hydrocarbon containing a substituted five-membered ring with one double bond (cyclopentene) with the following molecular formula of C6H10. C-H bonds are implied.
Answers
answer
H H
| |
H--C--C=C--C--H
| |
H H
steps
C6H10 Cyclopentene Skeleton
Draw a picture with 5 sides and 1 double line in the middle, and put a "C" at each corner.
Add an "H" atom to each corner that doesn't already have one.
Count how many "H" atoms you have and make sure it adds up to 10.
This is what the chemical cyclopentene looks like!
It's like drawing a picture of a shape and filling in the corners with crayons, but instead, we're using atoms to make a molecule that looks like a shape with a double line in the middle.
ChatGPT
a level chemistry

2
Select the correct answer
in a redex reaction, what folle does the reducing agent play?
OA. it gives up electrons
OB. it keeps electrons
OC. it takes electrons
OD. it takes onygen atoms
Answers
Answer:
A. it gives up electrons
Explanation:
In a redox reaction, the reducing agent is the element or compound that undergoes oxidation and gives up electrons. The oxidizing agent is the element or compound that undergoes reduction and gains electrons.
Hope that helps.
3.If two quantities are directly proportional, when one quantity increases by 10 percent, the other_____________.
Answers
If two quantities are directly proportional, when one quantity increases by 10 percent, the other quantity will also increase by 10 percent.
If two quantities are directly proportional, it means that they vary in such a way that a change in one quantity leads to a proportional change in the other quantity. In this scenario, if one quantity increases by 10 percent, the other quantity will also increase by 10 percent.
Let's consider two variables, x and y, that are directly proportional. Mathematically, we can represent this relationship as y = kx, where k is the constant of proportionality. When x increases by 10 percent, we can express it as (1 + 0.10)x = 1.10x.
Substituting this into the equation y = kx, we get y = k(1.10x). Since k is a constant, it remains unchanged. Therefore, the new value of y is 1.10 times the original value of y, which represents an increase of 10 percent.
To summarize, when one quantity increases by 10 percent in a directly proportional relationship, the other quantity also increases by 10 percent. This concept is fundamental in various fields such as physics, economics, and mathematics, where understanding direct proportionality is crucial for analyzing and predicting relationships between variables.
for more such questions on quantity
https://brainly.com/question/4804631
#SPJ8
What might happen if water molecules did not have a slight negative charge on one end and a slight positive charge on another
Answers
Water molecules did not have a slight negative charge on one end and a slight positive charge on another, the loss of polarity would have profound effects on various biological, chemical, and physical processes. The unique properties of water that are vital for life as we know it would be significantly altered, potentially rendering many biological systems nonfunctional and disrupting the stability of ecosystems.
Loss of hydrogen bonding: The polarity of water molecules allows them to form hydrogen bonds with each other and with other polar substances.Hydrogen bonds are relatively weak but essential for various biological processes, including protein folding, DNA structure, and the stabilization of cell membranes. Altered solubility: Water's polarity contributes to its excellent solvent properties. It can dissolve a wide range of substances, including salts, sugars, and polar molecules, due to its ability to surround and separate charged or polar particles. Changes in boiling and freezing points: The polarity of water affects its boiling and freezing points. Water has a relatively high boiling point and melting point compared to other substances of similar molecular weight. Altered surface tension: Surface tension is the cohesive force that holds the surface of a liquid together. Water exhibits relatively high surface tension due to the cohesive forces between water molecules resulting from their polarity. Changes in heat capacity: Water's ability to absorb and retain heat is crucial for temperature regulation in many organisms and helps moderate temperature changes in the environment.For such more question on Water molecules
https://brainly.com/question/21426318
#SPJ8
how many days does it take the earth to revolve around the Sun? this is for science class plz help.
Answers
Answer:
365 days = 1 year (to be exact)
if you add 25 grams of NaCO3 to 100 ml of .500 m HCl, which reactant will be limiting.
Answers
Taking into account the reaction stoichiometry, the reactant HCl is the limiting reagent.
Reaction stoichiometryIn first place, the balanced reaction is:
Na₂CO₃ + 2 HCl → 2 NaCl + CO₂ + H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Na₂CO₃: 1 moleHCl: 2 molesNaCl: 2 molesCO₂: 1 moleH₂O: 1 moleThe molar mass of the compounds is:
Na₂CO₃: 106 g/moleHCl: 36.45 g/moleNaCl: 58.45 g/moleCO₂: 44 g/moleH₂O: 18 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Na₂CO₃: 1 mole ×106 g/mole= 106 gramsHCl: 2 moles ×36.45 g/mole= 72.9 gramsNaCl: 2 moles ×58.45 g/mole= 116.9 gramsCO₂: 1 mole ×44 g/mole= 44 gramsH₂O: 1 mole ×18 g/mole= 18 gramsDefinition of molarityMolar concentration is a measure of the concentration of a solute in a solution, be it some molecular, ionic, or atomic species.
Molarity indicates the number of moles of solute that are dissolved in a given volume and it is calculated by:
molarity= number of moles÷ volume
Limiting reagentThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction.
Limiting reagent in this caseIn first place, you know:
Molarity of HCl= 0.500 MNumber of moles of HCl= ?Volume of HCl= 100 mL= 0.100 L (being 1000 mL= 1 L)Replacing in the definition of molarity:
0.500 M= number of moles÷ 0.100 L
Solving:
number of moles= 0.500 M× 0.100 L
number of moles= 0.05 moles
This indicates that 0.05 moles of HCl reacts.
To determine the limiting reagent, it is possible to use a simple rule of three as follows: if by stoichiometry 106 grams of Na₂CO₃ reacts with 2 moles of HCl, 25 grams of Na₂CO₃ reacts with how many moles of HCl?
moles of HCl= (25 grams of Na₂CO₃× 2 moles of HCl)÷ 106 grams of Na₂CO₃
moles of HCl= 0.4717 moles
But 0.4717 moles of HCl are not available, 0.05 moles are available. Since you have less moles than you need to react with 25 grams of Na₂CO₃, HCl will be the limiting reagent.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
Draw a plausible transition state for the bimolecular reaction of nitric oxide with ozone. Use dashed lines to indicate the atoms that are weakly linked together in the transition state.No(g) + o3(g) --> NO2(8) + O2(g)
Answers
One weakly bound intermediate is formed between the oxygen atom of O3 and one of the nitrogen atoms of NO, and another weakly bonded is formed between the oxygen atom of O3 and the nitrogen atom of NO.
Draw a plausible transition state for the bimolecular reaction of nitric oxide with ozone.The bond between nitrogen and oxygen in NO is partially broken, while the bond between the two oxygen atoms in O3 is also partially broken. The bonds between nitrogen and oxygen in NO2 and between the two oxygen atoms in O2 are partially formed.
How do ozone and nitric oxide interact?Nitric oxide and ozone then easily combine to form nitrogen dioxide and oxygen. No net ozone gain occurs as a result of the technique mentioned above. Concentrations are higher in the troposphere than can be explained by these processes alone.
To know more about the bimolecular reaction here;
https://brainly.com/question/1195122
#SPJ1
ch4+br2 ch3br+hbr which type of reaction does this equation represent
Answers
To solve such this we must know the concept of combination reaction. Therefore, the given reaction CH\(_4\)+Br\(_2\) \(\rightarrow\)CH\(_3\)Br+ HBr is a combination reaction.
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
CH\(_4\)+Br\(_2\) \(\rightarrow\)CH\(_3\)Br+ HBr
The above reaction is a combination reaction. In combination reaction, more than one reactant combine to form a product. Because new chemicals are created during combination reactions, they are often referred to as synthesis.
Therefore, the given reaction is a combination reaction.
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ1
The reactant concentration in a zero-order reaction was 8.00×10−2 M
after 140 s and 4.00×10−2 M after 400 s
. What is the rate constant for this reaction?
Answers
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1, depending on which rate was used to calculate it.
Determining the rate constantThe rate of the reaction is given by the equation:
Rate = -k[A]
where k is the rate constant and [A] is the concentration of the reactant.
Rate at t=140 s:
Rate = (8.00×10−2 M - 0 M) / (140 s - 0 s)
= 5.71×10−4 M/s
Rate at t=400 s:
Rate = (4.00×10−2 M - 0 M) / (400 s - 0 s)
= 1.00×10−4 M/s
Since this is a zero-order reaction, the rate of the reaction is constant, and we can use either rate to calculate the rate constant:
k = Rate / [A]
Using the rate at t=140 s:
k = 5.71×10−4 M/s / 8.00×10−2 M = 7.14×10−3 s−1
Using the rate at t=400 s:
k = 1.00×10−4 M/s / 4.00×10−2 M
= 2.50×10−3 s−1
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1.
Learn more on zero-order reaction https://brainly.com/question/21663229
#SPJ1
please help
Which of the following lists contains elements with seven electrons in their outermost energy levels?
He, Ne, and Ar
Cr, Mn, and Fe
Br, I, and Cl
O, S, and Se
Answers
Answer:
A)He, Ne, and Ar
Explanation:
I believe its this one. Sorry if its wrong. Remember that 8 electrons are in the outermost energy level .
A Compound X Consists of carbon 40%, hydrogen 6.7% and the rest being oxygen. If the relative molecular mass is 60 , determine its molecular formula.
Answers
The molecular formula of Compound X is C2H4O2.
To determine the molecular formula of Compound XFirst, we must identify the empirical formula. The empirical formula shows a compound's simplest whole-number atom distribution.
Let's assume we have 100 grams of compound X to begin with. We are able to operate with percentages as grams because to this presumption.
Convert the percentages into grams:
Carbon: 40% of 100g = 40g
Hydrogen: 6.7% of 100g = 6.7g
Oxygen: The remaining mass is oxygen, so it would be 100g - 40g - 6.7g = 53.3g
Convert the masses of each element into moles:
Carbon: 40g / molar mass of carbon = 40g / 12g/mol = 3.33 mol
Hydrogen: 6.7g / molar mass of hydrogen = 6.7g / 1g/mol = 6.7 mol
Oxygen: 53.3g / molar mass of oxygen = 53.3g / 16g/mol = 3.33 mol
Divide each number of moles by the smallest number of moles to obtain the simplest ratio:
Carbon: 3.33 mol / 3.33 mol = 1
Hydrogen: 6.7 mol / 3.33 mol ≈ 2
Oxygen: 3.33 mol / 3.33 mol = 1
The empirical formula is CH2O, representing the simplest ratio of atoms in Compound X.
Knowing the molar mass of the empirical formula (CH2O), which is computed as follows, is necessary to determine the molecular formula:
Carbon: 1 atom × molar mass of carbon = 1 × 12g/mol = 12g/mol
Hydrogen: 2 atoms × molar mass of hydrogen = 2 × 1g/mol = 2g/mol
Oxygen: 1 atom × molar mass of oxygen = 1 × 16g/mol = 16g/mol
Summing up the molar masses gives us 12g/mol + 2g/mol + 16g/mol = 30g/mol.
Since Compound X's relative molecular mass is specified as 60, we may get the proportion between that number and the empirical formula mass using the formula 60g/mol / 30g/mol = 2.
This means that the molecular formula of Compound X is a multiple of the empirical formula: (CH2O)2.
Therefore, the molecular formula of Compound X is C2H4O2.
Learn more about empirical formula here : brainly.com/question/1439914
#SPJ1
What forms of energy are produced when
fossil fuels burn?
Answers
When fossil fuels burn, several forms of energy are produced, including:
Heat energy: The primary form of energy released during fossil fuel combustion is heat. Fossil fuels contain chemical energy stored for millions of years, and when they burn, this energy is released in the form of heat. The heat energy can be harnessed for various purposes, such as heating buildings or generating steam to drive turbines.
Light energy: Burning fossil fuels can also produce light energy in the form of flames or glowing embers. This light energy is a byproduct of combustion.
Mechanical energy: Heat generated by burning fossil fuels can be converted into mechanical energy. This is typically achieved by using heat to produce steam, which drives a turbine connected to a generator. The rotating turbine converts the heat energy into mechanical energy, which is further transformed into electrical energy.
Electrical energy: Through the process described above, burning fossil fuels can ultimately generate electrical energy. The mechanical energy produced by the turbine is converted into electrical energy by the generator. Electrical energy can power various devices, appliances, industries, and infrastructure.
It's critical to note that while burning fossil fuels can produce useful forms of energy, it also results in the release of carbon dioxide and other greenhouse gases. This contributes to climate change and environmental concerns. As a result, there is a global shift towards cleaner and renewable energy sources to mitigate these negative impacts.
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
There are four molecules of nitrogen and nine molecules of hydrogen present in the diagram.
When the reaction is complete, how many molecules of NH3 are produced?
What is the limiting reactant?
How many molecules of each reactant are remain after the reaction is complete?
Answers
After the reaction is complete, no nitrogen and no hydrogen molecules remain, and 8.00 x 1014 molecules of NH3 are produced.
In the equation, nitrogen and hydrogen react at a high temperature, in the presence of a catalyst, to produce ammonia, according to the balanced chemical equation:N2(g)+3H2(g)⟶2NH3(g)The coefficients of each molecule suggest that one molecule of nitrogen reacts with three molecules of hydrogen to create two molecules of ammonia.
So, to determine how many molecules of ammonia are produced when four nitrogen and nine hydrogen molecules are present, we must first determine which of the two reactants is the limiting reactant.
To find the limiting reactant, the number of moles of each reactant present in the equation must be determined.
Calculations:
Nitrogen (N2) molecules = 4Hence, the number of moles of N2 = 4/6.02 x 1023 mol-1 = 6.64 x 10-24 mol
Hydrogen (H2) molecules = 9Hence, the number of moles of H2 = 9/6.02 x 1023 mol-1 = 1.50 x 10-23 mol
Now we have to calculate the number of moles of NH3 produced when the number of moles of nitrogen and hydrogen are known, i.e., mole ratio of N2 and H2 is 1:3.
The mole ratio of N2 to NH3 is 1:2; thus, for every 1 mole of N2 consumed, 2 moles of NH3 are produced.
The mole ratio of H2 to NH3 is 3:2; thus, for every 3 moles of H2 consumed, 2 moles of NH3 are produced.
From these mole ratios, it can be observed that the limiting reactant is nitrogen.
Calculation for NH3 production:
Nitrogen (N2) moles = 6.64 x 10-24 moles
The mole ratio of N2 to NH3 is 1:2; therefore, moles of NH3 produced is 2 × 6.64 × 10−24 = 1.33 × 10−23 moles.
Now, to determine how many molecules of NH3 are produced, we need to convert moles to molecules.
1 mole = 6.02 x 1023 molecules
Thus, 1.33 x 10-23 moles of NH3 = 8.00 x 1014 molecules of NH3 produced.
To find the amount of each reactant remaining after the reaction is complete, we must first determine how many moles of nitrogen are consumed, then how many moles of hydrogen are consumed, and then subtract these from the initial number of moles of each reactant.
The moles of nitrogen consumed = 4 moles × 1 mole/1 mole N2 × 2 mole NH3/1 mole N2 = 8 moles NH3
The moles of hydrogen consumed = 9 moles × 2 mole NH3/3 mole H2 × 2 mole NH3/1 mole N2 = 4 moles NH3
Thus, the moles of nitrogen remaining = 6.64 × 10−24 mol – 8 × 2/3 × 6.02 × 10^23 mol-1 = 5.06 × 10−24 mol
The moles of hydrogen remaining = 1.50 × 10−23 mol – 4 × 2/3 × 6.02 × 10^23 mol-1 = 8.77 × 10−24 mol
Finally, the number of molecules of each reactant remaining can be calculated as follows:
Number of N2 molecules remaining = 5.06 × 10−24 mol × 6.02 × 10^23 molecules/mol = 3.05 × 10−1 molecules ≈ 0 molecules
Number of H2 molecules remaining = 8.77 × 10−24 mol × 6.02 × 10^23 molecules/mol = 5.28 × 10−1 molecules ≈ 0 molecules.
For more such questions on molecules
https://brainly.com/question/24191825
#SPJ8
what is chemistry........
Answers
Answer:
the branch of science that deals with the identification of the substances of which matter is composed; the investigation of their properties and the ways in which they interact, combine, and change; and the use of these processes to form new substances.
Explanation:
I hope this helps!
Answer:
The branch of science that deals with the identification of the substances of which matter is composed; the investigation of their properties and the ways in which they interact, combine, and change; and the use of these processes to form new substances.
Explanation:
Chemistry is a branch of natural science that deals principally with the properties of substances, the changes they undergo, and the natural laws that describe these changes. The study of chemistry spans the range from qualitative in focus to quantitative.
The boiling point of an aqueous solution is 101.54 ∘C. What is the freezing point
Answers
Answer:
101.54 .C.
Explanation:
Which sample contains the largest number of oxygen atoms? Select one: a. 8.0 g of carbon dioxide b. 8.0 g of potassium chlorate c. 8.0 g of calcium perchlorate d. 8.0 g of sodium hydroxide
Answers
The sample with the largest number of oxygen atoms will be calcium perchlorate.
Number of atoms in a compoundSince we are not looking at the number of moles, the mass of the compounds has no bearing on the number of atoms of oxygen.
The chemical formula for carbon dioxide is \(CO_2\). Thus, it has 2 atoms of oxygen.The chemical formula for potassium chlorate is \(KClO_3\). Thus, it has 3 oxygen atoms.The chemical formula for calcium perchlorate is \(Ca(ClO_4)_2\). Thus, it has 8 atoms of oxygen.The chemical formula for sodium hydroxide is NaOH. Thus, it has 1 atom of oxygen.Therefore, the compound with the largest number of oxygen atoms is calcium perchlorate.
More on the number of atoms in compounds can be found here: https://brainly.com/question/1686912
#SPJ1
What mass of H₂ is required to produce 2 moles of NH3?
Answers
Answer: RATIOS IN TERMS OF MOLES
Solution: Solution According to the balanced equation, 2 moles of NH3 are produced when 3 moles of H2 react.
2 SO2(g) + O2(g) + 2 H2O(ℓ) −→ 2 H2SO4(ℓ)
What mass in grams of SO2 is needed to
react with 1527 g of O2?
Answers
Answer:
6116g
Explanation:
2SO2(g) + O2(g) + 2H2O(ℓ) −→ 2H2SO4(ℓ)
We want to find the mass in grams of SO2 that is needed to react with 1527 g of O2. First we must convert the grams of O2 to moles of O2 then to moles of SO2 and then to grams of SO2
So first lets find the molar mass of O2
The mass of oxygen according to a periodic table is 15.999
Using this the mass of O2 would be 15.999(2) = 31.988g
Next we need to identify the mole ratio of O2 to SO2
Looking at the equation for 1 mole of O2 there are two moles of SO2
Next we need to find the molar mass of SO2
Again the mass of oxygen is 15.999g and the mass of Sulfur is 32.066
So the mass of SO2 would be 15.999(2) + 32.066 = 64.064g
Now that we have found all the needed conversions :
1 mol O2 = 31.988g 1 mol O2 = 2 mol SO21 mol SO2 = 64.064gWe can now use dimensional analysis to calculate the answer.
Kindly check the attached image to see the table. ( sorry if its a bit blurry )
Explanation : The conversions are used to cancel out the units to get to the final unit which is gSO2.
Once the units are cancelled out except for the gSO2 we mutliply and divide based off of what the table says to do.
Here first we divide 1527 by 31.988. We than multiply by 2. Finally we multiply by 64.064 to get the final answer which is 6116gSO2
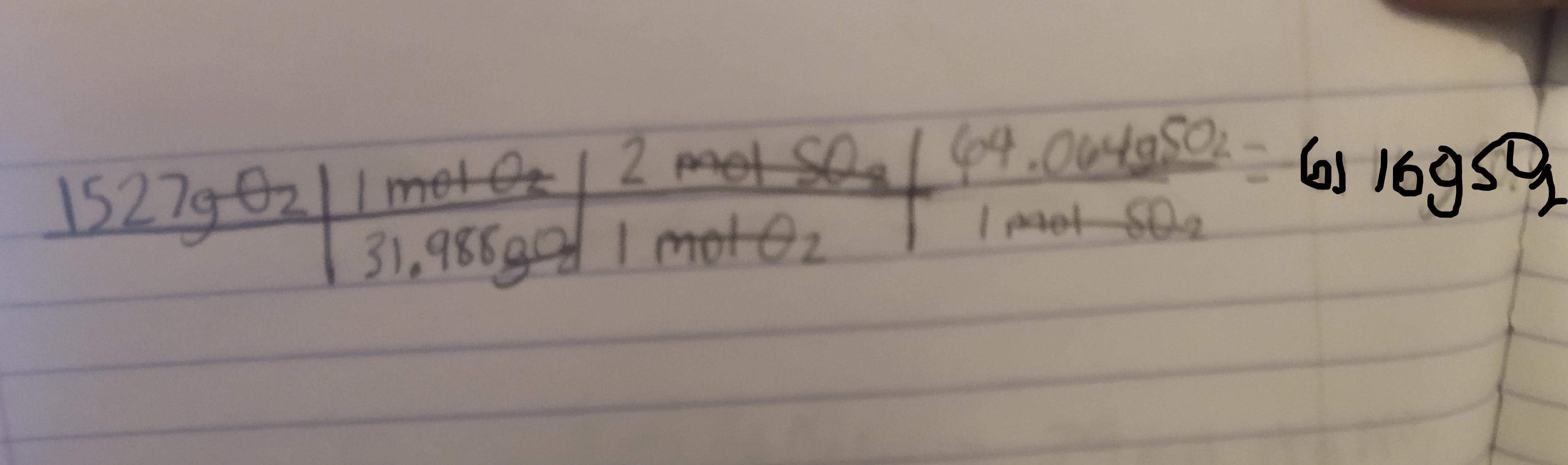
how many grams of CaO (mm=56 grams) would be produced from 25.2 grams of CaCo3 (MM=100 grams)
Answers
Explanation:
CaCO3 = CaO + CO2
1mol. 1 mol
100. 56
1 ×100. 1 × 56
100. 56
100g of CaCO3 produces 56g of CaO
25.2g of CaCO3 produces x
x = 25.2 ×56
100
x =14.112g of CaO
Why is the classification species not considered a group? (1 point)
O Each species is a separate type of organism.
O Each species is an individual organism.
O Each species lacks the characteristics of the levels above.
O Each species shares characteristics with other species.
Answers
Each species is a separate type of organism.
A species is a group of creatures that share similar traits. The same species of organisms are capable of sexual reproduction as well as interbreeding and producing fertile offspring. It is a fundamental unit of taxonomy and classification.The system is divided into seven categories: Kingdom, Phylum or Division, Class, Order, Family, Genus, and Species. Kingdom is the most inclusive category.In a group, many types of an organism can be included even if they do not share the same traits. But species is a group of organisms that share similar traits.For example, human beings are species as they are all alike in physical features, way of reproduction, etc. But the animal is considered a group because it included a variety of living beings.Therefore, Each species is not considered a group.
Learn more about taxonomy here:
https://brainly.com/question/1304906
#SPJ9
A 5 kg ball is traveling at the same speed as a 10 kg ball. Compared to with 5 kg ball, the 10 kg ball has (2 points)
Answers
Answer: twice the momentum
Explanation:
A solution has a pH of 12.1. What is the concentration of hydrogen ions? Hydroxide ions?
Answers
Taking into account the definition of pH and pOH, the concentration of hydrogen ions and hydroxide ions is 7.94×10⁻¹³ M and 0.0126 M respectively.
Definition of pH and pOHpH is a measure of acidity or alkalinity that indicates the amount of hydrogen ions present in a solution or substance.
The pH is defined as the negative base 10 logarithm of the activity of hydrogen ions, that is, the concentration of hydrogen ions or H₃O⁺:
pH= - log [H⁺]= - log [H₃O⁺]
Similarly, pOH is a measure of hydroxyl ions in a solution and is expressed as the logarithm of the concentration of OH⁻ ions, with the sign changed:
pOH= - log [OH⁻]
The following relationship can be established between pH and pOH:
pOH + pH= 14
The concentration of hydrogen ions and hydroxide ionsBeing pH= 12.1, the [H₃O⁺] is calculated as:
12.1= - log [H₃O⁺]
Solving:
[H₃O⁺]= 10⁻¹² ¹
[H₃O⁺]= 7.94×10⁻¹³ M
The concentration [H₃O⁺] is 7.94×10⁻¹³ M
Being pH= 12.1, pOH is calculated as:
pOH + 12.1= 14
pOH= 14 - 12.1
pOH= 1.9
Replacing in the definition of pOH the concentration of OH⁻ ions is obtained:
- log [OH⁻]= 1.9
Solving
[OH⁻]= 10⁻¹ ⁹
[OH⁻]= 0.0126 M
In summary, the [OH⁻] is 0.0126 M.
Learn more about pH and pOH:
brainly.com/question/16032912
#SPJ1
From the 2 enthalpy profile diagrams, state which
one is likely to represent this reaction 6C(s) + 6H2(g) + 3O2(g) → C6H12O6(s) Hf = -1086.4 kJ mol-1 and give a reason why.

Answers
Answer:
Diagram A
Explanation:
The information tells us that Hf = -1086.4 kJ mol-1. Since the value is negative, this tells us that the reaction is exothermic. In an exothermic reaction, energy is given out to the surroundings, thus the arrow must go from the reactants to the products to show the drop in enthalpy.
Could you guys help me on why I got this wrong

Answers
5.25moles is the moles of carbon monoxide. The mole notion is an easy way to express the amount of a substance.
The mole notion is an easy way to express the amount of a substance. Any measurement is divided into two parts: the numerical magnitude plus the units in which the magnitude is expressed. For example, if the weight of a ball is 2 kilogrammes, the magnitude is '2' and the unit is 'kilogramme'.
According to stoichiometry
moles of carbon monoxide = 1.75×3=5.25moles
Therefore, the correct option is option D.
To know more about mole, here:
https://brainly.com/question/30892840
#SPJ1
CH3-C_=CH + HCl ( 1:2) -->
Answers
3
C≡CH
2mole
HCl
A
CH
3
C(Cl)
2
CH
3
Heat
aq.KOH
B
CH
3
COCH
3
CHALLENGE The circles below represent of the large circle, and multiply it by 30. That Earth and the moon. Measure the diameter would be the correct distance from Earth to the moon at this scale. Draw the two circles in the space provided. Use the correct distance you found.● = Earth ●=moon

Answers
To draw the two circles, we would need to draw a smaller circle with a diameter of 2,532.5 miles (representing the moon) and a larger circle with a diameter of 75,974.4 miles (representing the Earth) that is 30 times larger than the smaller circle.
What is the explanation for the above response?If we assume that the larger circle represents the Earth, then the diameter of the Earth would be 30 times the diameter of the smaller circle representing the moon. Let's say that the diameter of the smaller circle is x. Then the diameter of the larger circle (Earth) would be 30 times x or 30x.
To find the correct distance from Earth to the moon at this scale, we need to know the actual distance from Earth to the moon, which is approximately 238,855 miles or 384,400 kilometers. If we divide this distance by the scale factor of 30, we get:
238,855 miles / 30 = 7,961.8 miles
Therefore, the diameter of the smaller circle (moon) would be approximately 7,961.8 miles / π = 2,532.5 miles (rounded to one decimal place). And the diameter of the larger circle (Earth) would be 30 times that or 75,974.4 miles
So, to draw the two circles, we would need to draw a smaller circle with a diameter of 2,532.5 miles (representing the moon) and a larger circle with a diameter of 75,974.4 miles (representing the Earth) that is 30 times larger than the smaller circle.
Learn more about Earth at:
https://brainly.com/question/19581790
#SPJ1