Answers
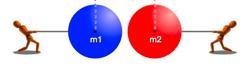
The tension in the rope that is fastened to the block is equivalent to the force F acting on the other rope in terms of magnitude.
What gravitational force has the most power?The mass of the Sun is significantly greater. Of all the things in our Solar System, it possesses the strongest gravitational pull. All eight planets are drawn into its center, where it maintains their orbits.
What celestial body in our solar system has the strongest gravitational pull?The Sun has the strongest gravitational force in our solar system since it is the largest and most massive object there. Every planet, as well as everything else, is drawn towards its centre of mass.
To know more about tension visit:-
https://brainly.com/question/11348644
#SPJ1
Related Questions
In which layer of the core sample would you most likely find fossil fuels?
A Layer X, on the surface of Earth
B Layer Z, deep in the earth
C Layer Y, close to the surface
D Layer X, Y, or Z, because fossil fuels are found everywhere
I chose _______ because ____________________________________________________________
Answers
Answer:
B Layer Z, deep in the earth
Explanation:
Fossils are found almost exclusively in sedimentary rocks-rocks that form when sand, silt, mud, and organic material settle out of water or air to form layers that are then compacted into rock.
30 example of redox reaction
Answers
1. Combustion of gasoline in a car engine
2. Rusting of iron
3. Photosynthesis in plants
4. Respiration in animals
5. Corrosion of metals
6. Bleaching of hair with hydrogen peroxide
7. Formation of ozone in the atmosphere
8. Electroplating of metals
9. Burning of wood
10. Reaction between bleach and ammonia
11. Reaction between copper and nitric acid
12. Reaction between iron and hydrochloric acid
13. Reaction between zinc and sulfuric acid
14. Reaction between magnesium and hydrochloric acid
15. Reaction between aluminum and hydrochloric acid
16. Reaction between sodium and water
17. Reaction between potassium and water
18. Reaction between lithium and water
19. Reaction between calcium and water
20. Reaction between barium and water
21. Reaction between copper and silver nitrate
22. Reaction between lead and silver nitrate
23. Reaction between zinc and copper sulfate
24. Reaction between iron and copper sulfate
25. Reaction between magnesium and copper sulfate
26. Reaction between aluminum and copper sulfate
27. Reaction between sodium and chlorine
28. Reaction between magnesium and chlorine
29. Reaction between aluminum and chlorine
30. Reaction between zinc and hydrochloric acid.
when 56.6 g of calcium and 30.5g of nitrogen gas under go a reaction that has 90% yield, what mass of calcium nitride is formed?
Answers
Answer:
152.57 g
Explanation:
The balanced chemical equation for the reaction between calcium and nitrogen gas to form calcium nitride is:
3Ca + N2 → Ca3N2
From the balanced equation, we can see that the mole ratio between calcium and calcium nitride is 3:1. This means that 3 moles of calcium react to form 1 mole of calcium nitride.
First, let's calculate the number of moles of calcium and nitrogen gas given:
Mass of calcium = 56.6 g
Molar mass of calcium (Ca) = 40.08 g/mol
Moles of calcium = Mass of calcium / Molar mass of calcium = 56.6 g / 40.08 g/mol = 1.41 mol
Mass of nitrogen gas = 30.5 g
Molar mass of nitrogen gas (N2) = 28.02 g/mol
Moles of nitrogen gas = Mass of nitrogen gas / Molar mass of nitrogen gas = 30.5 g / 28.02 g/mol = 1.09 mol
Since the reaction has a 90% yield, only 90% of the limiting reactant (which is calcium in this case) will be converted to product. Therefore, we need to multiply the moles of calcium by 0.90 to account for the yield:
Moles of calcium nitride formed = Moles of calcium x Yield = 1.41 mol x 0.90 = 1.27 mol
Now, using the mole ratio from the balanced equation, we can determine the mass of calcium nitride formed:
Molar mass of calcium nitride (Ca3N2) = 40.08 g/mol (molar mass of calcium) x 3 + 14.01 g/mol (molar mass of nitrogen) x 2 = 120.25 g/mol
Mass of calcium nitride formed = Moles of calcium nitride formed x Molar mass of calcium nitride = 1.27 mol x 120.25 g/mol = 152.57 g
So, the mass of calcium nitride formed in the reaction is 152.57 g.
Watts are a measurement of power
Answers
Answer:
Watts are a measurement of power, describing the rate at which electricity is being used at a specific moment. For example, a 15-watt LED light bulb draws 15 watts of electricity at any moment when turned on. Watt-hours are a measurement of energy, describing the total amount of electricity used over time.
Please mark as brainliest.
Answer: True
Watts are a measurement of power, used in light bulbs and other electricity. It is also measured as candela (C) in SI Units. The answer to the question is true because of this measurement.
Hope this helps!
A group claims that vaccines cause autism and children should not be
vaccinated. They claim that the symptoms of autism appear around the same
time children are vaccinated. Which piece of information is most useful in
evaluating this claim?
A. Famous people support the group.
B. Vaccines help prevent certain diseases.
C. The research study the group cites as evidence was proven to be
inaccurate.
OD. Vaccines used to contain ingredients that people thought could be
toxic.
Answers
The piece of information most useful in evaluating this claim is the research study the group cites as evidence was proven to be inaccurate (Option C).
Do Vaccines Cause Autism?A relatively recent publication indicated that certain vaccines might produce autism in children, thereby raising concerns about their use.
However, it has been shown that the scientific evidence in this publication may be erroneous and therefore the publication was retracted.
In conclusion, The piece of information most useful in evaluating this claim is the research study the group cites as evidence was proven to be inaccurate (Option C).
Learn more about vaccines here:
https://brainly.com/question/625596
#SPJ1
In Rutherford's Gold Foil Experiment, he discovered ? O neutrons O Protons O nucleus O electrons 4 5
Answers
Answer:
Rutherford's gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Based on these results, Rutherford proposed the nuclear model of the atom.
why does lead exist in a higher amount in brown algae than plankton?
Answers
Lead levels in plankton and algae are high, mostly as a result of environmental pollution brought on by human activity. While it is true that some brown algae species have the ability to accumulate heavy metals like lead.
Plankton and algae have high levels of lead, mostly as a result of environmental contamination brought on by human activities including mining, industrial operations, and the burning of fossil fuels.
Due to the fact that plankton and algae take up trace quantities of lead from the surrounding water, their tissues contain greater concentrations of the metal.
Learn more about brown algae, here:
https://brainly.com/question/31714795
#SPJ1
empirical formula for
Ca 40.078 22.3%
As 74.9216 41.6%
O 15.9994 35.6%
H 1.00794 0.560%
Answers
The empirical formula : CaAsO₄H
Further explanationThe empirical formula is the smallest comparison of atoms of compound =mole ratio of the components
The principle of determining empirical formula
Determine the mass ratio of the constituent elements of the compound. Determine the mole ratio by by dividing the percentage by the atomic mass Ca\(\tt \dfrac{22.3}{40.078}=0.556\)
As\(\tt \dfrac{41.6}{74.9246}=0.555\)
O\(\tt \dfrac{35.6}{15.9994}=2.225\)
H\(\tt \dfrac{0.56}{1.00794}=0.556\)
Divide by the smallest ratio(0.555) :
Ca : As : O : H ⇒
\(\tt \dfrac{0.556}{0.555}\div \dfrac{0.555}{0.555}\div \dfrac{2.225}{0.555}\div \dfrac{0.556}{0.555}=1\div 1\div 4\div 1\)
The chemical name for laughing gas is dinitrogen oxide. The two elements found in a sample of this gas are and .
Answers
Why would you not want to use a salt bridge saturated with potassium chloride solution in an electro-chemical cell made from a Ag/Ag+ cathode and a Cu/Cu²+ anode?
Answers
We can not use a salt bridge saturated with potassium chloride solution in an electro-chemical cell made from a Ag/Ag+ cathode and a Cu/Cu²+ anode because it will precipitate.
An electrochemical cell that uses a weak electrolyte and a salt bridge to connect oxidation as well as reduction half cells. A junction that joins the anodic with cathodic compartments of a cell and electrolytic solution is referred to as a salt bridge.
Because both chloride and potassium ions have very similar diffusion coefficients and minimise junction potential, the inactive minerals potassium chloride (KCl) frequently used. We can not use a salt bridge saturated with potassium chloride solution in an electro-chemical cell made from a Ag/Ag+ cathode and a Cu/Cu²+ anode because it will precipitate.
To know more about salt bridge, here:
https://brainly.com/question/2861410
#SPJ1
True or False:The following compound is an alkane:H3C-TrueFalseCH3Check it
Answers
Answer: CH3 is not an alkane. False
Explanation: The formula for alkane is given as:
\(\begin{gathered} C_n+H_{2n+2} \\ n:is\text{ }the\text{ }value\text{ }of\text{ }the\text{ }subscript \end{gathered}\)(c) What is the volume of 4 kg of water in liters?
Answers
Answer:
\(\huge\boxed{\sf v = 0.004\ m\³}\)
Explanation:
Given Data:Mass = m = 4 kg
Density of water = 1000 kg/m³ (Standard)
Required:Volume = v = ?
Formula:Density = m/v
Solution:1000 = 4 / v
Multiply v to both sides1000 × v = 4
Divide both sides by 1000v = 4/1000
v = 0.004 m³
\(\rule[225]{225}{2}\)
An error during which cellular process would create a gene mutation?
Answers
An error during DNA replication would create a gene mutation.
During DNA replication, the genetic information in a cell is copied to make new DNA molecules. However, mistakes can occur during this process, leading to changes in the DNA sequence, which can result in a mutation. Mutations can also be caused by exposure to environmental factors, such as radiation or chemicals, which can damage the DNA molecule directly or affect the cellular processes involved in DNA replication.
Mutations can have a variety of effects on the organism, ranging from no effect to causing serious health problems or even death. Gene mutations can also be inherited from a parent, which can result in genetic disorders or predisposition to certain diseases. Therefore, it is important to understand the mechanisms of gene mutations and their potential impacts on organisms.
To know more about the Gene mutation, here
https://brainly.com/question/15448555
#SPJ1
WHICH STATEMENTS DESCRIBE DENSITY
1.Can be used to identify and unknown substance.
2.Is a physical property of matter
3.Can be measured with a balance
4.Is the amount of space an object takes up
5.Can be measured with a graduated cylinder or a ruler
6.Is the amount of matter in a certain amount of space
7.Is used to determine density
8.May be calculated using data from a balance and a graduated cylinder
9.Is the amount of matter a substance has
WHICH OF THE STATEMENTS ABOVE DESCRIBE MASS
WHICH OF THE STATEMENTS ABOVE DESCRIBE VOLUME
Answers
Explanation:
Density - 6, 8Mass - 1, 2, 3, 9, 7Volume - 4, 5, 7.hope this helps you.
At which temperature can water contain the most
dissolved sugar?
Answers
Answer:
At 10ºC or 50ºF water can cotain the most dissolved sugar.
Burning 2.00 mol of hydrogen releases 483.6 kJ of energy. Determine how much energy, in kilojoules, must be supplied to convert 3.00 mol of water vapor into hydrogen gas and oxygen gas.
Express your answer with the appropriate units.
Answers
The amount of heat energy (in KJ) needed to convert 3 moles of water vapor into hydrogen gas and oxygen gas is 1450.8 KJ
Balanced equationWe'll begin by writing the balanced equation. This is illustrated below:
2H₂ + O₂ --> 2H₂O ΔH = 483.6 KJ
From the balanced equation above,
2 moles of water (H₂O) required 483.6 KJ to produce hydrogen gas and oxygen gas
How to determine the heat energy needed to convert 3 moles of water to hydrogen gas and oxygen gasThe heat energy needed to convert 3 moles of water can be obtained as illustrated below:
From the balanced equation above,
2 moles of water (H₂O) required 483.6 KJ to produce hydrogen gas and oxygen gas
Therefore,
3 moles of water (H₂O) will require = 3 × 483.6 = 1450.8 KJ to produce hydrogen gas and oxygen gas
Thus, we can conclude that the energy needed to convert 3 moles of water is 1450.8 KJ
Learn more enthalpy change:
https://brainly.com/question/11967318
#SPJ1
How much ice in grams would have to melt to lower the temperature of 352 mL
of water from 15 ∘C
to 0 ∘C
? (Assume that the density of water is 1.0 g/mL
Answers
Answer:
66 grams of ice would have to melt to lower the temperature of 352 mL of water from 15 °C to 0 °C.
Explanation:
To calculate the amount of ice that would have to melt to lower the temperature of 352 mL of water from 15 °C to 0 °C, we need to use the formula:
Q = m_water * c_water * ΔT_water + m_ice * Lf
where,
Q = the amount of heat transferred,
m_water = the mass of water, c_water is the specific heat capacity of water,
ΔT_water = the change in temperature of water, m_ice = the mass of ice,
Lf = the specific latent heat of fusion of ice.
First, let's calculate the amount of heat transferred to the water:
Q = m_water * c_water * ΔT_water
Q = 352 g * 1.0 cal/(g*°C) * (15-0) °C
Q = 5,280 cal
Next, we can use the specific latent heat of fusion of ice, which is 80 cal/g, to calculate the amount of heat required to melt the ice:
Q = m_ice * Lf
Q = m_ice * 80 cal/g
m_ice = Q / Lf
m_ice = 5,280 cal / 80 cal/g
m_ice = 66 g
write the chemical equation of the reaction with a change in colour
Answers
Answer:
(i) Change in colour (ii) Change in temperature (iii) Formation of precipitate
Explanation:
(i) Change in colour: Reaction between lead nitrate solution and potassium iodide solution. Pb(NO3)2(aq)+2KI → PbI2(s)+2KNO3(aq) In this reaction, colour changes from colourless to yellow. (ii)Change in temperature: Action of dilute sulphuric acid on zinc. Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2 In this reaction, heat is evolved (iii) Formation of precipitate: Action of barium chloride on sodium sulphate. BaCl2(aq) +Na2SO4(aq) → BaSO4(s) +2NaCl(aq) BaSO4(s)
Alpha Decay
1. If an atom of Uranium-238 decays via alpha emission, what would be its daughter product? (You will need a periodic table.)
2. If an atom of Thorium -228 decayed via beta emission, what would be the daughter isotope?
Answers
Thorium 234 is the daughter product of Uranium-238 decays whereas protactinium 234 is the daughter product of Thorium -228.
What are the products of Uranium-238 and Thorium -228?If an atom of Uranium-238 decays via alpha emission, a nucleus of uranium 238 decays by alpha emission to form a daughter nucleus, thorium 234 while If an atom of Thorium -228 decayed via beta emission, the daughter isotope is protactinium 234.
So we can conclude that thorium 234 is the daughter product of Uranium-238 decays whereas protactinium 234 is the daughter product of Thorium -228.
Learn more about isotope here: https://brainly.com/question/14220416
#SPJ1
13. A nuclear power plant is normally next to a body of water so that
a. it can help warm marine life in the water if the water gets cold.
b. the water can be used to cool down the nuclear reactor.
c. it can be used to reduce costs of operation
d. the body of water can provide a beautiful view of the power plant.
Answers
Answer: B
Explanation: nuclear reactor's are built near water because the water helps convey heat.
what do the water, nitrogen, and carbon cycle all have in common
Answers
Answer:
How are the water carbon and nitrogen cycles related?
The three main cycles of an ecosystem are the water cycle, the carbon cycle and the nitrogen cycle. These three cycles working in balance are responsible for carrying away waste materials and replenishing the ecosystem with the nutrients necessary to sustain life.
Explanation:
The water, nitrogen, and carbon cycle involve the exchange of gases with the atmosphere.
What are biogeochemical cycles?Biogeochemical cycles can be described as the movement of nutrients and other elements between biotic factors and abiotic factors. The matter on Earth is conserved since matter can neither be created nor destroyed, so it is recycled in the system in various forms.
The major elements include in Biogeochemical cycles are Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, and Sulphur. These elements are recycled via the biotic and abiotic components of the ecosystem.
Types of Biogeochemical Cycles are basically divided into two types: Gaseous cycles include the Carbon cycle, the Oxygen cycle, the Nitrogen cycle, and the Water cycle. Sedimentary cycles include the Sulfur cycle, the Phosphorus cycle, and the Rock cycle.
In the water, nitrogen, and carbon cycle the exchange of water nitrogen gas, and carbon with the atmosphere.
Learn more about Biogeochemical Cycles, here:
https://brainly.com/question/1204069
#SPJ2
What is the significance of temperature t
Answers
Answer:
send a pic or more info
Explanation:
A solution is prepared at that is initially in diethylamine , a weak base with , and in diethylammonium chloride . Calculate the pH of the solution. Round your answer to decimal places.
Answers
Answer:
10.96
Explanation:
A solution is prepared at 25 °C that is initially 0.14 M in diethylamine, a weak base with Kb = 1.3 × 10⁻³, and 0.20 M in diethylammonium chloride. Calculate the pH of the solution. Round your answer to 2 decimal places.
Step 1: Calculate the pOH of the solution
Diethylamine is a weak base and diethylammonium (from diethylammonium chloride) its conjugate acid. Thus, they form a buffer system. We can calculate the pOH of this buffer system using the Henderson-Hasselbach's equation.
pOH = pKb + log [acid]/[base]
pOH = -log 1.3 × 10⁻³ + log 0.20 M/0.14 M
pOH = 3.04
Step 2: Calculate the pH of the solution
We will use the following expression.
pH + pOH = 14
pH = 14 - pOH = 14 -3.04 = 10.96
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
I take in oxgyen from the air and send it into your bloodstream. I also remove the carbon dioxide from your body. What system am I?
muscular system
respiratory system
digestive system
skeletal system please help
Answers
What does it mean that something is a conservative ion?
Answers
Answer:
The major ionic constituents whose concentrations can be determined from the salinity are known as conservative substances. Their constant relative concentrations are due to the large amounts of these species in the oceans in comparison t
4- Calculate the mol fraction of ethanol and water in
a sample of rectified spirit which contains 95% of
ethanol by mass.
Answers
Answer:
math si hard
Explanation:
The mole fration of ethanol and water in a sample of rectified spirit which contains 95% of ethanol by mass is 0.8 and 0.11.
How do we calculate mole fraction?Mole fraction of any substance will be calculated by dividing the moles of that substance from the total moles of the solution.
Moles (n) will be calculated as:
n = W/M, where
W = given mass
M = molar mass
Given that 95% of ethanol by mass is present in the sample, so 95g of ethanol is present in 100g of solution.
Mass of solvent (water) = 100 - 95 = 5g
Moles of water = 5g / 18g/mol = 0.27mol
Moles of ethanol = 95g / 46g/mol = 2.06mol
Mole fraction of water = 0.27 / (0.27+2.06) = 0.11
Mole fraction of ethanol = 2.06 / (0.27+2.06) = 0.8
Hence required mole fraction of water & ethanol is 00.11 and 0.8 respectively.
To know more about mole fraction, visit the below link:
https://brainly.com/question/1601411
#SPJ2
Please help me make analysis scheme flow chart for the detection of cation present in my unknown salt mixture of Ag+, Pb2+, or Ca2+ cations based on their solubility in the given reagents.
I can only use these reagents: Na2CO3, NaCl, & Na2SO4
The suspected cations in the unknown cation mixture are: Ag+, Pb2+, or Ca2+.
I used 0.5 M solution of all these reagents.
My test results are:
Na2CO3: 20 drops of sodium carbonate were used and silver blue precipitate was produced.
NaCl: 20 drops of sodium chloride were used and no precipitate was formed.
Na2SO4: 20 drops of sodium sulfate were used yellow precipitate was produced.
Please please help me make analysis flow chart based on this information and also tell me in what sequence these reagents will be added to unknown salt mixture.
I suspect that the cation may be Ca2+ based on solubility rules. But I'm not sure.
Answers
The possible action your solution might contain is amongst \(Ag^+\), \(Pb^{2+}\), or \(Ca^{2+}\).
What are the solutions?A solution is a homogeneous mixture of one or more solutes dissolved in a solvent.
NaCl: 20 drops of NaCl were used and no precipitate was formed.
No precipitation formed means the \(Cl^-\) cation is soluble in water. From your chart, you might see the halide of \(Ag^+\) and \(Pb^{2+}\) is insoluble in water, which means they should have formed precipitation but you didn't. This means there's less possibility it contains \(Ag^+\) or \(Pb^{2+}\), so we are left with only \(Ca^{2+}\). Let's confirm it with the rest.
\(Na_2SO_4\) : 20 drops of \(Na_2SO_4\) were used yellow precipitate was produced.
Even though all other sulfates form precipitation ( Insoluble in water)
\(Na_2CO_3\) : 20 drops of sodium carbonate were used and the silver-blue precipitate was produced.
It seems all of their Carbonate is insoluble in nature. ( which is our case too ).
From the above result, only the reaction with NaCl was deemed to be decisive and it indeed contains \(Ca^{2+}\) ions. Means you are correct.
Learn more about the solutions here:
https://brainly.com/question/1616939
#SPJ1
Draw all structural and geometric isomers of butene and name them
Answers
Alkene is an unsaturated hydrocarbon which contain at least one carbon-carbon double bond. Here butene is an alkene with the chemical formula C₄H₈. The functional group present in alkenes is the double bond.
The structural isomers are those isomers in which the atoms are completely arranged in a different order but have the same molecular formulas. It is also called the constitutional isomers.
Geometric isomers are two or more compounds with the same number and types of atoms and bonds but have different geometries for the atoms.
To know more about Geometric isomers, visit;
https://brainly.com/question/28188244
#SPJ1
Which sentence from the article shows Clara Ma's MAIN opinion about the qualities of good scientists? (A ) In the decade since I did my very first science project, I have wondered a lot about what it takes to be a good scientist (B) To me, what makes a truly good scientist is also what makes a good person. (C) We should take the time to consider other people's opinions, even when they conflict with our own. (D) Of course, good scientists are driven by curiosity, but curiosity does not have to be limited to science,
Answers
Answer:
The answer is B.
Explanation: