Answers
Answer:
It is one of the covalent bonds in which the electrons are shared equally; therefore, dipole moment exists between the atoms in a molecule and there is no charge separation between the atoms in a molecule.
Answer: A covalent bond in which the electrons are shared equally
Explanation:
A P E X
Related Questions
Explain, using the terms "energy state" "electrons" and "light" how the colors of a flame test are produced .
Answers
The terms "energy state" "electrons" and "light" colors of a flame test are produced is because excitement of electron caused by the increased temprature
The color observed during the flame test result from the excitement of the electron caused by the increased temprature and the electron jumps from their ground state to a higher energy level and as they return to their ground state they emit visible light and the flame test is an analytical procedure used in chemistry to detect the presence of certain element and primarily metal ion and based on each element characteristics emission spectrum the color of flames in general also depend on temprature and oxygen and it sees the different color in the flame
Know more about colors
https://brainly.com/question/8039067
#SPJ1
if two substance are at the same temperature, their enthalpy
Answers
Answer:
cannot be measure
Hope this helps :) !!!
What is the density of a piece of granite whose volume is 20 mL and mass is 53
grams?
3.05 g/mL
2.75 g/mL
4.0 g/mL
2.65 g/mL
Answers
2.65g/ml is the density of a piece of granite whose volume is 20 mL and mass is 53grams. Density is the mass of a specific material per unit volume.
What is density?Density is the mass of a specific material per unit volume. Density is defined as d = M/V, in which d represents density, M is weight, as well as V is volume. Density is generally expressed in grams every cubic centimetre. Water, for example, has a density of 1 gram per square centimeter, but Earth has a density of 5.51 kilograms per cubic centimetre.
Density is sometimes measured in kilos per cubic centimeter (in metre-kilogram-second or SI units). The density of air, for example, is 1.2 kilos per cubic metre.
density = mass / volume
=53/ 20
=2.65g/ml
Therefore, 2.65g/ml is the density of a piece of granite whose volume is 20 mL and mass is 53grams.
To learn more about density, here:
https://brainly.com/question/13434141
#SPJ1
How do the valence electrons of an atom affect chemical reactions?
Answers
Valence electrons are the electrons in the outermost shell of an atom and are responsible for chemical reactions. In a chemical reaction, atoms gain or lose electrons to achieve a stable electron configuration, which is known as the octet rule. The number of valence electrons an atom has determines its chemical reactivity and how it will bond with other atoms. For example, atoms with only a few valence electrons, such as hydrogen, are highly reactive and will readily form chemical bonds, while atoms with many valence electrons, such as noble gases, are relatively unreactive and do not easily form chemical bonds.
Chlorofluorocarbons are ?
A. colorless, odorless gases that prevent red blood cells from carrying oxygen to the body
B. man-made chemicals containing chlorine and fluorine that cause
ozone molecules to break down
C. chemicals produced in factories that are used to prevent air
pollution
D. molecules containing chlorine and fluorine that block UV radiation
from reaching the Earth
Answers
Chlorofluorocarbons (CFCs) are synthetic compounds that contain chlorine, fluorine, and carbon. They were widely used in the past as refrigerants, propellants in aerosol products, and foam-blowing agents. CFCs have been found to have a detrimental effect on the Earth's ozone layer when released into the atmosphere. They can reach the stratosphere, where they undergo a chemical reaction facilitated by ultraviolet (UV) radiation, resulting in the release of chlorine atoms. These chlorine atoms then participate in a destructive cycle that breaks down ozone molecules, leading to ozone depletion. Due to their harmful impact on the ozone layer, the production and use of CFCs have been phased out or regulated under international agreements like the Montreal Protocol to protect the Earth's ozone layer.
Chlorofluorocarbons (CFCs) are man-made chemicals containing chlorine and fluorine that cause ozone molecules to break down. Thus, option B is the answer.
Chlorofluorocarbons are non-toxic, synthetic compounds that contain atoms of Chlorine, Fluorine and Carbon. They are commonly used in the manufacture of aerosol sprays and are also used as solvents and refrigerants. CFCs were first introduced in 1928 by General Motors Company for its refrigerators.
While CFCs are very safe to use in most applications and are stable in the lower atmosphere, these chemicals when released to the upper atmosphere can cause significant reactions. CFCs when released into the upper atmosphere can lead to the destruction of the ozone molecules followed by the release of the UV radiation into the atmosphere.
Thus, CFCs are man-made chemicals which cause ozone molecules to break down.
Learn more Chlorofluorocarbons, here:
https://brainly.com/question/1393491
When you decide whether or not the data supports the original hypothesis, you are
O creating a theory
forming a hypothesis
O contributing to the body of knowledge
stating a law
O asking questions
Answers
Contributing to the body of knowledge.
When you decide whether or not the data supports the original hypothesis, you are "contributing to the body of knowledge." Explanation:Scientific investigation is a way of answering questions about the natural world. An inquiry or investigation can be initiated by a researcher or a group of researchers who have questions regarding a certain phenomenon. The inquiry or investigation is usually done by conducting an experiment or making an observation and then collecting data. After collecting the data, the researchers analyze it to check if it supports their hypothesis or not.The body of knowledge refers to the totality of data that has been collected and analyzed. It comprises all the data that researchers have acquired over time through scientific investigations. When researchers decide whether or not the data supports their original hypothesis, they contribute to this body of knowledge. The new data may either confirm the hypothesis or lead to a revision or rejection of it, adding to the body of knowledge.
for more questions on knowledge
https://brainly.com/question/29610548
#SPJ8
Given the following balanced equation:
3Cu(s) + 8HNO3(aq) = 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(l)
Determine the mass of copper (II) nitrate that would be formed from the complete reaction
of 35.5g of copper with an excess of nitric acid.
Answers
Answer: The mass of copper (II) nitrate produced is 105.04 g.
Explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
\(\text{Number of moles}=\frac{\text{Given mass}}{\text{Molar mass}}\) ......(1)
Given mass of copper = 35.5 g
Molar mass of copper = 63.5 g/mol
Plugging values in equation 1:
\(\text{Moles of copper}=\frac{35.5g}{63.5g/mol}=0.560 mol\)
The given chemical equation follows:
\(3Cu(s)+8HNO_3(aq)\rightarrow 3Cu(NO_3)_2(aq)+2NO(g)+4H_2O(l)\)
By the stoichiometry of the reaction:
If 3 moles of copper produces 3 moles of copper (II) nitrate
So, 0.560 moles of copper will produce = \(\frac{3}{3}\times 0.560=0.560mol\) of copper (II) nitrate
Molar mass of copper (II) nitrate = 187.56 g/mol
Plugging values in equation 1:
\(\text{Mass of copper (II) nitrate}=(0.560mol\times 187.56g/mol)=105.04g\)
Hence, the mass of copper (II) nitrate produced is 105.04 g.
write the molecular formula for each listed compound:
dinitrogen trioxide & nitrogen monoxide hydrochloric acid & chloric
acid sulfuric acid & sulfurous acid
Answers
Answer:
dinitrogen trioxide = N2O3
nitrogen monoxide = NO
hydrochloric acid = HCl
chloric acid = HClO3
sulfuric acid = H2SO4
sulfurous acid = H2SO3
1. Before starting, make a prediction: If substances B and C are both in the gas phase and are at the same energy level, which of the two substances will need to have more energy transferred out in order to change to the liquid phase? Substance B or substance C? Explain your answer.
Answers
Answer:
Substance C
Explanation:
Substance C would be the answer because Substance C has a lower attraction level. Because of this, it takes more energy to take out of in order to become a liquid.
The AH° of the reaction is -1367 kJ. Calculate the work done on the system at 25°C. C2H5OH(1) + 302(g) → 2CO2(g) + 3H₂O(0)
Answers
The work done on the system for the reaction \(C2H5OH(1) + 3O2(g) → 2CO2(g) + 3H2O(0)\) at 25°C is 1240 kJ.
The work done on a system can be calculated using the Gibbs free energy equation: \(\beta ΔG = ΔH - TΔS,\) where ΔH is the enthalpy change, T is the temperature, and ΔS is the entropy change. The given enthalpy change is -1367 kJ, which represents the amount of heat released during the reaction.
To calculate the work done on the system at 25°C, we need to calculate the entropy change.
\(ΔS°\)(C₂H₅OH) = 160.7 J/K·mol
ΔS°(O₂) = 205.0 J/K·mol
ΔS°(CO₂) = 213.8 J/K·mol
ΔS°(H₂O) = 188.8 J/K·mol
Using the formula \(ΔS° = ΣnS°(products) - ΣmS°(reactants)\), we can calculate the entropy change for the reaction. This gives:
\(ΔS° = [2(213.8 J/K·mol) + 3(188.8 J/K·mol)] - [1(160.7 J/K·mol) + 1(205.0 J/K·mol) + 3(188.8 J/K·mol)]\)
\(ΔS° = -470.9 J/K·mol\)
Now we can calculate the work done on the system using the Gibbs free energy equation:
\(ΔG = ΔH - TΔS\)
\(ΔG = -1367 kJ - (25°C + 273.15)K × (-0.4709 kJ/K·mol)\)
\(ΔG = -1240 kJ/mol\)
The negative sign indicates that the work is done on the system, and its magnitude is 1240 kJ. Therefore, the work done on the system for the reaction \(C2H5OH(1) + 3O2(g) → 2CO2(g) + 3H2O(0)\) at 25°C is 1240 kJ.
To learn more about Gibbs free energy here
https://brainly.com/question/13318988
#SPJ1
HELLLP MEEEE
Write the chemical formula for each of the given compounds.

Answers
Answer:
sodium perchorate is NaCLO4
calcium sulfite CaSO3
potassium hydroxide KOH
lithium nitrate LiNO3
Explanation:
sodium perchorate has one Sodium
one Chlorine and 4 Oxygen
calcium sulfite has one Calcium on sulfate and 3Oxygen
lithium nitrate has one Lithium oneNitrogen
3 Oxygen
Predict the products in the chemical reaction, Na+AlN
Answers
If germanium, has an electron configuration by shell of 2 8 18 4, in what orbitals are the valence electrons?
Check all that apply.
If germanium, has an electron configuration by shell of 2 8 18 4, in what orbitals are the valence electrons?Check all that apply.
1s
1
s
2s
2
s
2p
2
p
3s
3
s
3p
3
p
3d
3
d
4s
4
s
4p
4
p
4d
4
d
4f
4
f
Answers
Based on the electronic configuration of Germanium which is 1s²2s²2p⁶3s²3p⁶3d¹⁰4s²4p², the orbitals containing the valence electrons are the 4s and 4p orbitals.
What is electronic configuration?Electronic configuration is the arrangements of electrons in orbits or electron shells around the nucleus of an atom.
The electronic configuration an atom describes the number of electrons present in the atom as well as the number of valence electrons in the atom.
The electronic configuration of atoms serves as the basis of the periodic table where elements with the same number of valence electrons belong to the same group and elements with the same number of of electron shells are found in the same period.
The electronic configuration of Germanium is as follows:
1s²2s²2p⁶3s²3p⁶3d¹⁰4s²4p²From the above electronic configuration, the valence electrons of germanium are found in the 4s and 4p orbitals.
Learn more about electronic configuration at: https://brainly.com/question/26084288
#SPJ1
Which of the following is not an example of potential energy? Group of answer choices electrical energy, chemical energy, gravitational energy, elastic energy.
PLS HELP FAST
Answers
Answer: The one listed below that's NOT an example of potential energy is mechanical energy. Mechanical energy is categorized as a kinetic energy with light, sound, and thermal/heat energy.
HOPE THIS HELPS
The energy that a body possesses as a result of its position with relation to other bodies, internal tensions, electric charge, and other considerations . Mechanical energy is not an example of potential energy.
What is potential energy ?Potential energy is a form of stored energy that is dependent on the relationship between different system components. When a spring is compressed or stretched, its potential energy increases.
Although a force must be applied to an object in order for it to store potential energy, potential energy is technically stored inside matter.
The potential energy must be released, despite the fact that the energy is held in the object's mass due to gravity or elastic forces.
Thus, Mechanical energy is not an example of potential energy.
To learn more about potential energy refer the link below;
https://brainly.com/question/24284560
#SPJ2
what are the formulas of the salts that precipitate when the reaction mixture iscooled?2.why does the alkyl halide layer switch from the top layer to the bottom layer at thepoint where water is used to extract the organic layer?3.an ether and an alkene are formed as by-products in this reaction. draw the struc-tures of these by-products and give mechanisms for their formation.
Answers
The formula of the salt is R-X + NaOH → R-ONa + H₂O; the alkyl halide layer switches from the top layer to the bottom layer due to its lower density than water; and the by-products formed are an ether and an alkene.
1. The reaction mixture is cooled to cause the salts to precipitate. The formula of the salts is dependent on the reactants used in the reaction. In this case, the reaction of an alkyl halide with an aqueous solution of sodium hydroxide gives the sodium salt of the alkyl halide plus water. The chemical formula of this salt is R-X + NaOH → R-ONa + H2O.
2. When the reaction mixture is cooled, the alkyl halide layer switches from the top layer to the bottom layer because the alkyl halide is less dense than water. This causes the alkyl halide to sink to the bottom of the mixture, forming a separate layer from the aqueous layer above.
3. An ether and an alkene are formed as by-products in this reaction. The ether is formed from the dehydration of the alkyl alcohol, which is formed when the alkyl halide reacts with the sodium hydroxide.
To learn more about alkene visit:
https://brainly.com/question/10113466
#SPJ4
Complete Question:
1. What are the formulas of the salts that precipitates when the reaction mixture is cooled?
2. Why does the alkyl halide layer switch from the top layer to the bottom layer at the point where water is used to extract the organic layer?
3. An ether and an alkene are formed as by-products in the reaction. Draw the structures of these by-products and give mechanisms for their formation.

characteristics. of. rusting
Answers
Answer: metal turn orange and weaker as it gets oxidised
Explanation:
3-4. When something interests you, how do you make a hypothesis?
5-6. Why is it important to take a good notes when you are conducting an experiment?
7-8. What is the last step in the scientific method? Explain.
9-10. Name one thing you want to investigate this year for a scientific project? Why?

Answers
Answer:
3-4. I think we should research the topic, and make a assumption based on what you learned.
5-6.There are times when a scientist is unable to answer his own question. If he has taken good notes, another scientist may come along later and use his notes to find the answer. Every year there is new knowledge. The scientific method is a step-by-step process.
7-8.The final step of the scientific process is to report your results. Scientists generally report their results in scientific journals, where each report has been checked over and verified by other scientists in a process called peer review.
9-10. I don't know this one.
I did it wrong but I just can’t figure it out.


Answers
(a) The number of moles of Ag⁺ that reacted is 0.05 mmoles and moles of Cl- that reacted is 0.05 mmoles.
(b) The number of moles of AgCl(s) formed is 0.05 moles
(c) The molarity of Ag⁺ after the reaction = 0.0 M
(d) The molarity of NO₃⁻ after the reaction is 0.25 M
What is the number of moles of Ag and Cl- that reacted?The number of moles of Ag and Cl- that reacted is calculated as follows from the equation of the reaction:
AgNO₃ (aq) + NaCl (aq) ---> AgCl (s) + NaNO₃ (aq)
(a) The number of moles of Ag and Cl- that reacted:
Moles of Ag⁺ = 0.5 * 0.1
Moles of Ag⁺ = 0.05 mmoles
Moles of Ag⁺ that reacted = 0.05 mmoles
Moles of Cl⁻ = 0.5 * 0.1
Moles of Cl⁻ = 0.05 mmoles
Moles of Cl⁻ that reacted = 0.05 mmoles
(b) The number of moles of AgCl(s) formed.
Moles of AgCl that formed = 0.05 moles
(c) Since there are no more Ag⁺ ions in the mixture, the molarity of Ag⁺ after the reaction = 0 moles
(d) The molarity of NO₃⁻ after the reaction.
Moles of NO₃⁻ = 0.5 * 0.1
Moles of NO₃⁻ = 0.05 moles
Volume of mixture = 0.2 mL
The molarity of NO₃⁻ after the reaction = 0.05/0.2
The molarity of NO₃⁻ after the reaction = 0.25 M
Learn more about molarity at: https://brainly.com/question/30404105
#SPJ1
How much heat is required to raise the temperature of 67.0g of water from 25.7°C to
66.0°C? The specific heat of H₂O is 4.184J/g°C)
a) 40.3 kJ
b) 11.3 kJ
c) 67.0 kJ
d) 280.3 kJ
e) 2.70 kJ
Answers
The amount of heat required to raise the temperature of 67 g of water from 25.7°C to 66°C is approximately equal to 40.3 kJ. Thus, the answer is option a) 40.3 kJ.
How do you calculate the amount of heat required to raise the temperature of the water to 66.0°C?The amount of heat required to raise the temperature of a substance is given by the equation:
q = m * c * ΔT
Here, q is the amount of heat required to raise the temperature , m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature due to the heat supplied.
Substituting the given values in the equation, we get:
q = (67 g) * (4.184 J/g°C) * (66°C - 25.7°C)
q = 40,332 J
Converting this value to kilojoules we obtain:
q = 40.332 kJ = 40.3 kJ
Thus 40.3 kJ of heat energy is required to raise the temperature of 67g of water from 25.7 °C to 66° C.
Learn more about specific heat capacity here:
https://brainly.com/question/29766819
#SPJ1
The following two organic compounds are structural isomers to each other. Carefully identify and justify the structural isomers type (skeletal, functional, or positional) with their common molecular formula

Answers
Structural isomers are molecules with the same molecular formula but with different structural formulae. This means that they have the same number and types of atoms, but they are arranged differently. The following two organic compounds are structural isomers of each other.
Carefully identify and justify the structural isomers type (skeletal, functional, or positional) with their common molecular formula.Common molecular formula: C6H14Structural isomers:(i) Hexane: Hexane is a straight-chain alkane with six carbon atoms and no double bonds or rings. The carbon atoms are linked together in a linear or straight-chain configuration in the skeletal isomer. The skeletal isomer differs in terms of the arrangement of atoms in its molecule. This indicates that it is a skeletal isomer.(ii) 2-methylpentane: It is a branched-chain alkane with six carbon atoms and no double bonds or rings. It differs from the first molecule in terms of the location of a methyl group on the second carbon of the five-carbon chain, rather than a straight six-carbon chain. This difference is due to a change in the positioning of the carbon atoms in the molecule. As a result, it is a positional isomer, as it differs by the position of the functional group or substituent. Therefore, the skeletal and positional isomerism types are present between these two compounds.For such more question on molecular
https://brainly.com/question/24191825
#SPJ8
5. Rust (Fe2O3) is produced when iron (Fe) reacts with oxygen (O2). Calculate the number
of grams of rust produced when 12 gram of iron react with oxygen.
Answers
Answer:
mole ratio = Fe:O2 = 2 : 3
36 grams of iron = how many moles?
well, 36/56 = 0.64 moles
multiply this by 1.5 =0.96 moles
this * 16 =15.43 grams
According to stoichiometry and balanced chemical equation of iron and oxygen 17.15 g of rust is produced when 12 g iron reacts with oxygen.
What is stoichiometry?Stoichiometry is the determination of proportions of elements or compounds in a chemical reaction. The related relations are based on law of conservation of mass and law of combining weights and volumes.
Stoichiometry is used in quantitative analysis for measuring concentrations of substances present in the sample.
The balanced chemical equation for formation of rust when iron reacts with oxygen is,
4 Fe + 3 O₂\(\rightarrow\) 2 Fe₂O₃
223.36 g of iron produces 319.38 g of rust
∴ 12 g iron will produce 12×319.38/223.36= 17.15 g of rust.
Therefore, 17.15 g rust is produced from 12 g iron when it reacts with oxygen.
Learn more about stoichiometry,here:
https://brainly.com/question/28780091
#SPJ2
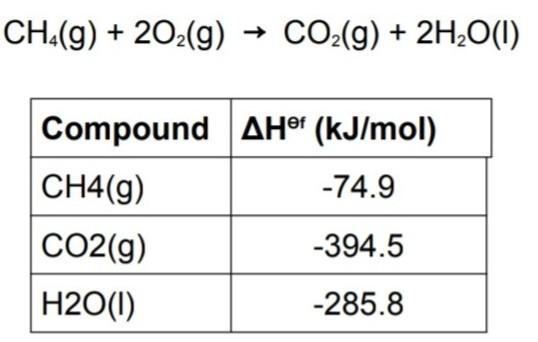
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
When dipentyl ether is treated with HI, what type of reaction occurs? both SN1 and SN2 SN2 E1 SN1 E2
Answers
Answer:
SN2
Explanation:
The first step of ether cleavage is the protonation of the ether since ROH is a better leaving group than RO-.
The second step of the reaction may proceed by either SN1 or SN2 mechanism depending on the structure of the ether. Methyl and primary ethers react with HI by SN2 mechanism while tertiary ethers react with HI by SN1 mechanism. Secondary ethers react with HI by a mixture of both mechanisms.
Dipentyl ether is a primary ether hence when treated with HI, the reaction with HI proceeds by SN2 mechanism as explained above.
A canister of gas contains a mixture of gases: Oxygen, 5.19 atm; nitrogen, 4.05 atm; and argon, 0.05 atm. What is the total pressure in the canister?
Answers
9.29 atm
From Dalton’s law total pressure, P equals sum of the partial pressures.
P =P1 + P2 + P3 + .....
P = 5.16 + 4.05 + 0.05
P = 9.29 atm
What volume of 2.00 M NaOH must be added into the 200.0 ml of 1.00M glycolic acid solution to make a buffer solution with pH = 4.15?
Answers
We need to add 100.0 mL of 2.00 M NaOH to the 200.0 mL of 1.00 M glycolic acid solution to make a buffer solution with pH = 4.15.
What is the volume needed for the buffer solution?To make a buffer solution with pH = 4.15 using glycolic acid and sodium hydroxide, we need to calculate the amount of NaOH needed to react with the glycolic acid and form the corresponding buffer.
The pKa of glycolic acid is 3.83. Therefore, we need to use the Henderson-Hasselbalch equation to calculate the ratio of the concentrations of the conjugate base (glycolate) to the acid (glycolic acid) needed to achieve the desired pH:
pH = pKa + log([A-]/[HA])
4.15 = 3.83 + log([A-]/[HA])
0.32 = log([A-]/[HA])
10^0.32 = [A-]/[HA]
2.04 = [A-]/[HA]
Now we can use the equation for the balanced chemical reaction between glycolic acid and sodium hydroxide:
HOCH2COOH + NaOH → HOCH2COO-Na+ + H2O
We can see that one mole of glycolic acid reacts with one mole of NaOH, so we need to calculate the number of moles of glycolic acid in the 200.0 ml of 1.00M solution:
n(HA) = C(HA) x V(HA)
n(HA) = 1.00 mol/L x 0.200 L
n(HA) = 0.200 mol
Since we need a 2.04:1 ratio of [A-]/[HA], we need to calculate the number of moles of glycolate (A-) needed:
n(A-) = 2.04 x n(HA)
n(A-) = 2.04 x 0.200 mol
n(A-) = 0.408 mol
Now we can calculate the volume of 2.00 M NaOH needed to react with this amount of glycolic acid:
n(NaOH) = n(HA) = n(A-)
n(NaOH) = 0.200 mol
V(NaOH) = n(NaOH) / C(NaOH)
V(NaOH) = 0.200 mol / 2.00 mol/L
V(NaOH) = 0.100 L = 100.0 mL
Learn more about buffer solution here: https://brainly.com/question/27371101
#SPJ1
Sea turtles use the earths geomagnetic filed to navigate their way home. What is the reason earth has a geomagnetic filed?
Answers
Answer:
Due to the iron inside the earth.
Explanation:
Imagine a sphere of Iron, as big as two-third the size of the moon and as hot as 5700 Kelvin. That is the Earth’s core.
The iron core isn't in its liquid form even at that temperature because it is crushed under immense gravity. This core is surrounded by 2000 km of other metals like iron and nickel which are in their molten state.
The temperature is not the same at every point in this molten layer. The hotter and less dense matter rises up, and the warm denser matter sinks. This causes convectional currents in the interior of the Earth.
Because of the Earth’s spin, there is a force that is established called the Coriolis force which causes swirling whirlpools here too.
This flow of molten metals produces electric currents which generate self-sustaining magnetic fields. And as a result of the Coriolis force, all these combined effects add up to produce one big magnetic field engulfing the Earth aligned in one direction.
A mountain has a height of 2.74 miles. How high is the mountain in meters? Use the fact that 1 mi=1.609 km .
Answers
kkknskndcnndcks
a
Answer:
Explanation:
20 Points to first correct answer! Identify the missing coefficient in the balanced equation and classify the type of reaction. Cl2O5 + H2O ⟶ ___HClO3 1; Combination 1; Decomposition 2; Combination 2; Decomposition
Answers
Answer:
.Combination
Explanation:
Answer:
actually i am pretty sure that the answer is decomposition 1
A compound with a molecular mass of 44.0 grams is found to be 81.82% carbon
and 18.18% hydrogen by mass. Finds its molecular formula. (HINT: Once you get
the mole to mole ratio you will need to multiple both by 3)
Answers
The molecular formula of the compound containing 81.82% carbon
and 18.18% hydrogen by mass is C₃H₈
From the question given above, the following data were obtained:
Carbon (C) = 81.82%
Hydrogen (H) = 18.18%
Molar mass of compound = 44 g/mol
Molecular formula =?We'll begin by calculating the empirical formula of the compound. This can be obtained as follow:
Carbon (C) = 81.82%
Hydrogen (H) = 18.18%
Empirical formula =?Divide by their molar mass
C = 81.82 / 12 = 6.818
H = 18.18 / 1 = 18.18
Divide by the smallest
C = 6.818 / 6.818 = 1
H = 18.18 / 6.818 = 2.67
Multiply by 3 to express in whole number
C = 1 × 3 = 3
H = 2.67 × 3 = 8
Thus, the empirical formula of the compound is C₃H₈
Finally, we shall determine the molecular formula of the compound. This can be obtained as follow:
Molecular formula = Empirical formula × n = molar mass
[C₃H₈]n = 44
[(12×3) + (1×4)]n = 44
[36 + 4]n = 44
40n = 44
Divide both side by 40
n = 44/40
n ≈ 1
Molecular formula = C₃H₈ × n
Molecular formula = C₃H₈ × 1
Molecular formula = C₃H₈Therefore, the molecular formula of the compound is C₃H₈
Learn more: https://brainly.com/question/13208888
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8