Which object has a mass of 27 grams. If each object has an identical *
volume of 3cm', which object has a mass of 27 grams
Answers
9 g/cm³ object has a mass of 27 grams.
Define mass?A substance or object's mass is a measurement of how much matter there is in it. Smaller masses can be measured in grams instead of kilograms (kg), which is the standard SI unit for mass (g).The kilogram is the kilogram, the standard international unit of mass (kg). Science and technology define a body's weight as the force that causes it to accelerate at a rate equal to the local acceleration of free fall in a given reference frame.The quantity of substance present in any item or body serves as the best definition of mass. We see mass in everything around us. For instance, objects like a table, chair, bed, football, glass, and even air have mass. Having said that, all things are light or heavy because of their mass.To learn more about mass refer to:
https://brainly.com/question/19385703
#SPJ1
Related Questions
If we increase the temperature of the vessel to 450 K at constant volume, what would the pressure inside the vessel be? a. 10 atm
b. 5 atm
c. 20 atm
d. 15 atm
Answers
The pressure inside the vessel would be 15 atm if we increased the temperature of the vessel to 450 K at constant volume. As a result, Option D is right.
According to the Ideal Gas Law, the pressure inside the vessel is directly proportional to the temperature (Kelvin) and the number of moles of gas present in the vessel, if the volume is kept constant. In other words, if we increase the temperature of the gas, the pressure inside the vessel will also increase.
The Ideal Gas Law equation is:
PV = nRTWhere:
P is the pressureV is the volumen is the number of molesR is the ideal gas constant (8.31 J/mol*K)T is the temperature in KelvinGiven that V is constant, we can rearrange the equation to solve for P:
P = (nRT)/V.Assuming that n and V are constants, then P is directly proportional to T. So, if the temperature increases by a factor of T2/T1, the pressure will increase by the same factor.
Let's say the initial pressure was 10 atm, and the initial temperature was 400 K. Then P1/T1 = 10 atm/400 K.
When the temperature is increased to 450 K, the pressure will become:
P2 = (P1 * T2)/T1 P2 = (10 atm * 450 K) / 400 K P2 = 15 atmSo, when the temperature of the vessel is increased to 450 K at constant volume, the pressure inside the vessel will increase, and the answer is "d. 15 atm".
Learn more about gauge pressure here: brainly.com/question/13095072
#SPJ4
suppose the sample of magnesium used in this lab was contaminated with another metal that does not react with hydrochloric acid. how would this have changed your results?
Answers
If the sample of magnesium used in a lab was contaminated with another metal that doesn't react with hydrochloric acid, then the results obtained in the experiment would be affected.
This is because the data collected during the experiment would reflect the reaction between hydrochloric acid and the contaminated sample instead of pure magnesium. As a result, the following changes in results might have been observed:
1. The mass of the contaminated sample would be higher than the mass of pure magnesium.
2. The rate of reaction between the contaminated sample and hydrochloric acid would be slower than the reaction between pure magnesium and hydrochloric acid.
3. The volume of hydrogen gas collected from the reaction would be lower than the volume of hydrogen gas collected in the reaction between pure magnesium and hydrochloric acid.
learn more about contaminated here
https://brainly.com/question/465199
#SPJ11
in the lab, we used two solvents, one for the extraction and one for the recrystallization. what solvent did we use for the extraction, and which for the recrystallization? what solvent is trimyristin more soluble in, and why?
Answers
The solvent we use for extraction is diethyl ether and the solvent we use for the recrystallization is acetone.
Trimyristin is more soluble in diethyl ether rather than in acetone. In order to get extracted from the nutmeg, Trimyristin has to be soluble in the solvent.
Why is Trimyristin more soluble in diethyl ether?
Trimyristin was extracted in the ether rather than acetone since the molecule is predominantly nonpolar and more soluble in the latter. Trimyristin was re-crystallized in acetone since acetone is more polar than ether, which indicates that trimyristin won't dissolve as well in acetone.Trimyristin can be easily dissolved by diethyl ether, a somewhat organic material, more readily than by an aqueous solvent. It can be more easily dissolved by acetone, an organic solvent, at a much higher temperature than by diethyl ether.To learn more about extraction and recrystallization visit:
https://brainly.com/question/12910778
#SPJ4
cho hỗn hợp gồm alcl3 và fecl3 trình bày cách tách từng muối riêng ra khỏi hỗn hợp
Answers
Answer:
ném NaOH vào thì th AlCl3 tan hết còn lại kết tủa Fe(OH)3 lần.
Lọc kết tủa riêng phần dung dịch tan riêng
Rồi Cho HCl vô lại là xong
Explanation:
In a fractionating column, what process is caused by heating?
Answers
Answer:
During the fractional distillation of crude oil: heated crude oil enters a tall fractionating column , which is hot at the bottom and gets cooler towards the top. vapours from the oil rise through the column. vapours condense when they become cool enough.
Hope it helps you :)
The fractionating column's crystal clear glass beads provide such a vast surface area for heated vapors to cool and condensed continuously.
The fractionating column is inserted into the mouth of the distillation flask, which contains the saturated solution to be separated.
Heated crude oil is pumped into a tall fractionating column that is heated at the bottom and cools as it rises.
The oil fumes ascend through the column. When vapors cool down sufficiently, they condense. Various heights are used to lead liquids out of the columns.
Learn more:
https://brainly.com/question/25671371?referrer=searchResults
How many lone pairs are in SO4 2-
Answers
Answer:
None
Explanation:
There are two S=O. bonds and two S-O bonds in sulfate ion lewis structure. Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. There are no lone pairs in the last shell of sulfur atom.
when working with acids, which of the following is the proper way to dilute these chemicals? group of answer choices place acid in a graduated cylinder then add water to the correct volume none of the above add water to the acid in a beaker add the acid to water
Answers
Adding the acid to water is the proper way to dilute chemicals. Begin by measuring the correct volume of acid in a graduated cylinder. Next, pour the acid into a beaker containing the correct volume of water. Finally, stir the solution until it is fully mixed.
What are acids?Acids are strong chemical compounds. When working with acids, it is important to dilute them in the correct manner to prevent harm to oneself or the surrounding environment.
The correct method of dilution for acids is to add the acid to water, not the other way around. This is because adding water to acid can cause an exothermic reaction that releases heat and may cause the acid to splash and burn you.
When diluting acids, be sure to add the acid to water slowly and stir continuously to prevent splashing and heat generation. Therefore, the correct answer is to add the acid to water.
Learn more about Acids here:
https://brainly.com/question/29796621
#SPJ11
How does the type of medium affect a sound wave?
Answers
sound waves travel faster in solids than in liquids, and faster in liquids than in gasses. While the density of a medium also affects the speed of sound, the elastic properties have a greater influence on the wave speed.
hope this helped
most imine formation reactions are performed in the presence of molecular sieves or magnesium sulfate. what is the purpose of these added reagents?
Answers
The purpose of using molecular sieves or magnesium sulfate in imine formation reactions is to remove any water present in the reaction mixture.
This is important because imine formation reactions require a dehydration step, which means water can interfere with the reaction and reduce the yield of the desired product. Molecular sieves and magnesium sulfate are both excellent drying agents that can remove water from the reaction mixture, thereby promoting the formation of imines. Therefore, they are added reagents used to ensure that the reaction proceeds efficiently and yields the desired product.
This is a telltale sign that the solution has dried completely. An organic solution will clump up when a drying agent, such MgSO4 magnesium sulfate, is first applied because it absorbs water. But if more drying agent is added, it will finally begin to move freely inside the solution like a powder. This is a visible cue that the organic solution has been appropriately dried and that the drying agent has been supplied in sufficient amounts.
Learn more about drying agent here
https://brainly.com/question/30712002
#SPJ11
what happens to water in the atmosphere after evaporation occurs
Answers
Answer:
Cloud form
Explanation:
Evaporation happens when a liquid substance becomes a gas. When water is heated, it evaporates. The molecules move and vibrate so quickly that they escape into the atmosphere as molecules of water vapor. ... Once water evaporates, it also helps form clouds.
___________________________________________________________
*rate this answer and say thanks please!
now you will investigate the emission spectra for a different element, helium. helium is the next element after hydrogen on the periodic table and has two electrons. do you think the emission spectra for an atom with two electrons instead of one will be significantly different than that of hydrogen? explain your answer.
Answers
The electron configuration of Helium (He) is 1s², which means that it has two electrons in its outermost shell.
Helium is an inert gas and, like hydrogen, it also emits a line spectrum when it is energized.Helium has a more complex spectrum than hydrogen because it has more electrons.
As a result, it emits more lines than hydrogen. Helium has two electrons, which implies that it will have twice the number of lines than hydrogen.
The emission spectrum of helium will have more lines than that of hydrogen because helium has more electrons.
To know more about electron configuration click on below link:
https://brainly.com/question/29757010#
#SPJ11
Formation of enolate (why it occurs)
Answers
Enolate formation occurs due to the removal of an acidic α-hydrogen from a carbonyl compound, resulting in the formation of a resonance-stabilized anion.
An acidic -hydrogen that is present on a carbonyl molecule, such as a ketone or an aldehyde, causes enolate production. A resonance-stabilized enolate anion is created when a strong base, such as sodium hydroxide or potassium hydroxide, is introduced.
This removes the acidic -hydrogen. This anion has a negative charge on the oxygen atom, which is stabilised by resonance, and a double bond between the carbon and oxygen atoms. Many organic processes, including aldol condensation, Michael addition, and Claisen condensation, include the intermediate step of enolate production.
Learn more about carbonyl:
https://brainly.com/question/26736570
#SPJ4
Describe the water cycle using the following words: precipitation, evaporation, condensation, and runoff.
Use 2-4 sentences and proper conventions (spelling, grammar, punctuation, etc.) to respond. Put all answers in your own words.
(help ! I'll give brainliest to the best paragraph answer)
Answers
With the characteristics of the states of matter and the transformation processes, it is possible to find the result for the description of the water cycle:
Water changes state on the planet but it is not lost, it is only transformed from one state to another: solid, liquid and gas.
The water cycle or hydrological cycle are all the changes that water has in a closed cycle.
The water in the liquid state is heated by the sun, which causes a part of the water to transform into gas through the evaporation process, these gas particles are very small therefore the pressure of the air makes them rise, as they rise through the atmosphere the temperature drops, therefore with the condensation process they pass to the liquid state.
The small drops of the atmosphere are joining them until their weight makes them fall towards the earth due to the rain process if the temperature is intermediate, if the temperature is extremely low the drops can pass to the solid state through the process of solidification falling as snow and ice.
When it is liquid water and it is soldered on the land, it can have a process of infiltration in the land and rocks called runoff and reach either the seas or underground levels where it is stored.
We can see that in this water is not lost only, it is transformed from one state to another, but the total amount of water remains constant.
Learn more about the water cycle here: brainly.com/question/2430469
It's #9 Pls help will give 100 points and brainiest
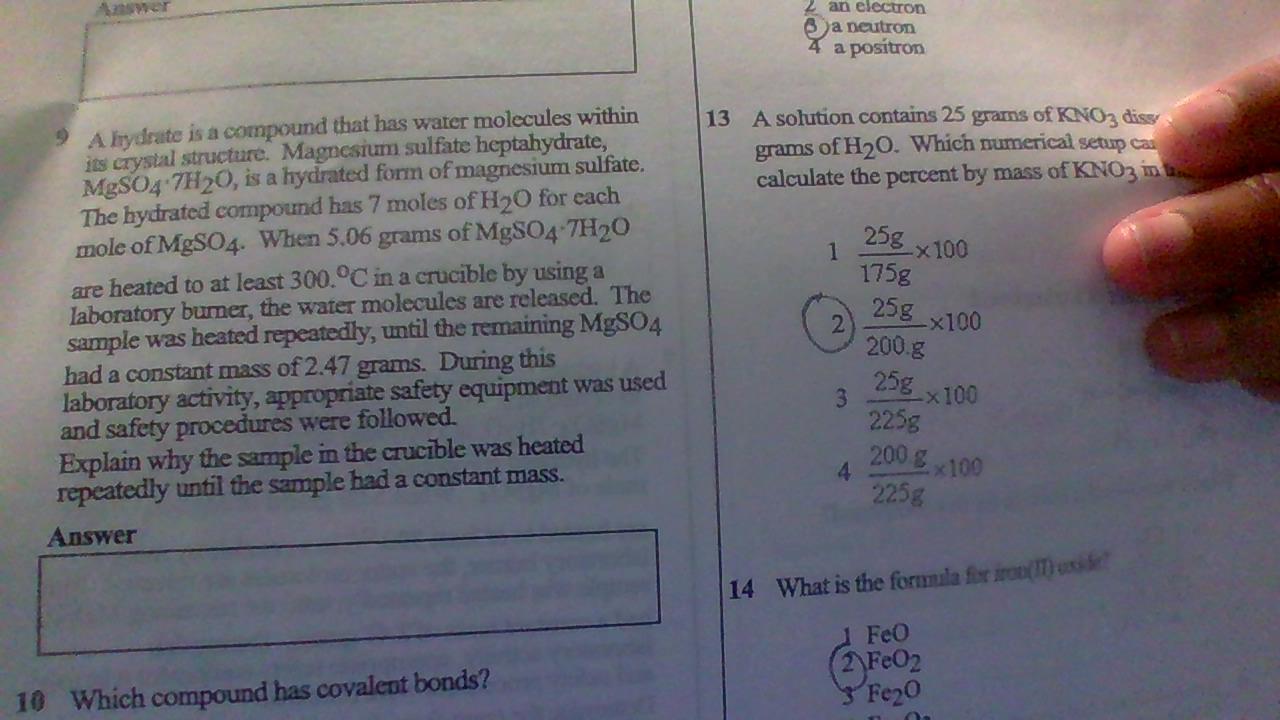
Answers
heating is repeated to ensure all water molecules have evaporated
Mass%
Mass of element/Mass of solution×10025/175+25×10025/200×10025/212.5%Option B
4. Calculate the molar mass of a mole of iodine, I2. Round to 2 decimal places.
Answers
The molar mass of I2 : 283.81 g/mol
Further explanationGiven
I2 compound
Required
The molar mass
Solution
Relative atomic mass (Ar) of element : the average atomic mass of its isotopes
Relative molecular weight (M) : The sum of the relative atomic mass of Ar
M AxBy = (x.Ar A + y. Ar B)
So for I2 :
= 2 x Ar I
= 2 x 126.90447 g/mol
= 253. 809 g/mol
= 253.81 g/mol
determine how much heat (in kj) of 2.89 mol of tio2(s)
Answers
Total heat generated by 2 mole of TiO2(s) is 4.963kJ.
The amount of heat released in the reaction of 2.89 mol of TiO2(s) can be calculated using the following equation: q = nCΔT, where n is the number of moles, C is the specific heat capacity of TiO2, and ΔT is the change in temperature.
The specific heat capacity of TiO2 is 683. 697. J/kgK. and the change in temperature is is 25k. By plugging in the values and converting J to kJ,
q = 2.89 * 25 * 683.697
=> 4963.35
In brief, the amount of heat released by 2.89 mol of TiO2(s) is 4.963kJ.
To know more about specific heat capacity click on below link:
https://brainly.com/question/16952828#
#SPJ11
Complete question :
determine how much heat (in kj) of 2.89 mol of tio2(s) with a temperature difference of 25k
What is the effective viscosity of water in pa∙s? given this information, does your answer to question 11 make sense? why or why not?
Answers
Water has a dynamic viscosity of 8.90 104 Pa.s.
The fluid's effective viscosity and average fluid velocity are equivalent. During fluid flow through the circular capillary, both change spatially.
The effective viscosity value is a single representative viscosity value for fluid flow under a certain set of conditions, much like the average velocity value. viscosity is the ability of a fluid (liquid or gas) to resist changing its shape or allowing nearby parts to move in relation to one another. Viscosity is a sign of flow resistance.
The fluidity, a metric indicating the ease of flow, is the reciprocal of viscosity. For instance, molasses is more viscous than water.
Learn more about viscosity here brainly.com/question/8736248
#SPJ4.
LEWIS DOT
Mg3 N2 how do I get my answer
Answers
Answer:
mayori moriti
Explanation:
dot al time
mlojwkWhat is the density of an object if it’s mass is 88.69g and it’s volume is 24.5mL?
Answers
Using the phase diagram for H2O what phase is water in at 1 atm pressure and -5C

Answers
The phase diagram of water depicts the behavior of water with respect to temperature and pressure, showing the physical states of water: solid, liquid, and gas, at different points on the diagram. It is also known as the pressure-temperature phase diagram
Water’s phase diagram has three phases, ice (solid), water (liquid), and steam (gas), which exist in equilibrium at the normal atmospheric pressure of one atmosphere (1 atm).At 1 atm pressure and -5°C, water is in a solid state, which is ice. The horizontal line on the diagram at 1 atm represents the normal atmospheric pressure on earth, while the vertical line at -5°C depicts the temperature point where the phase transition between water and ice occurs. The intersection of the horizontal and vertical lines indicates the phase of water at that specific temperature and pressure. When water is heated at 1 atm, its temperature increases until it reaches 100°C, where it boils and turns into steam (gas). Similarly, when water is cooled, its temperature decreases until it reaches 0°C, where it freezes and becomes ice (solid).When water is at 1 atm and at a temperature between 0°C and 100°C, it exists in a liquid state. If the temperature and pressure change, the physical state of water changes as well. Hence, the phase diagram of water helps us understand the behavior of water at different temperatures and pressures.
For such more question on temperature
https://brainly.com/question/27944554
#SPJ8
What is a mole in chemistry definition?
Answers
In chemistry, a mole is a unit of measurement used to express the amount of a substance, equal to 6.022 × 10²³ particles (such as atoms or molecules) of that substance.
The mole is used to relate the mass of a substance to the number of its constituent particles, allowing chemists to make quantitative predictions about chemical reactions. For example, the molar mass of a substance is the mass of one mole of that substance, expressed in grams. This allows chemists to calculate the amount of a substance needed for a reaction, or the amount produced in a reaction, based on the stoichiometry of the reaction. The mole concept is fundamental to many areas of chemistry, including stoichiometry, thermodynamics, and chemical kinetics, and is a key tool for understanding the behavior of matter at the molecular level.
Learn more about mole here:
https://brainly.com/question/26416088
#SPJ4
Which factor plays the biggest role in delaying the detection of childhood
diseases?
Answers
Answer:
poor access to health care providers
Explanation:
without health care providers you cant get tested.
which is a unique characteristic of the bonding between metal atoms?
Answers
Fe + N -> Fe2N balanced reaction
Answers
The balanced chemical equation for the reaction between iron (Fe) and nitrogen (N) to form iron nitride (Fe2N) is: 6 Fe + N2 → 2 Fe2N
What is the balanced chemical reaction?This equation is balanced because there are equal numbers of atoms of each element on both sides of the arrow, and the ratio of the reactants and products is 6:1 for Fe and N2, and 2:1 for Fe2N.
To balance the equation, we need to make sure that the number of atoms of each element is the same on both sides of the equation. Here's how we can do it:
On the LHS, we have 6 atoms of Fe and 2 atoms of N (since N2 consists of 2 nitrogen atoms bonded together).
On the RHS, we have 4 atoms of Fe (2 atoms in each Fe2N molecule) and 2 atoms of N (1 atom in each Fe2N molecule).
To balance the equation, we can multiply the reactants by 3 to get 6 Fe atoms and 6 N atoms:
6 Fe + 3 N2 → 2 Fe2N
Learn more about balanced reaction here: https://brainly.com/question/26694427
#SPJ1
why does no one love me? when will i feel appreciated?
Answers
Answer:
I appreciate you- owo... and people do love you. uwu. even tho I am a stranger I love you :D. ❤
You're the best person anyone could ever ask for. Trust me you're enough <3
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
Which of the following is an amorphous solid?
O
A. Diamond
B. Graphite
O C. Glass
O D. Iron
Answers
Answer:
C. Glass
Explanation:
Amorphous solids have a non-crystalline structure and no order. In that case, Diamonds, Graphite, and Iron all have a crystalline structure and order. You are left with C as your answer.
compare and contrast the similarities and differences between passive and active transport
Answers
A compound is composed of 53.33%carbon, 11.11%hydrogen and 35.56%oxygen. If the molecular mass of the compound is 90, what is the molecular formula of this compound?
Answers
Hope this helps :)

The temperature of a plasma is often ________ compared to the temperatures of gases, liquids, or solids.
Answers
The temperature of a plasma is often higher compared to the temperatures of gases, liquids, or solids.
Plasma is a state of matter that exists at very high temperatures, typically in the range of thousands to millions of degrees Celsius.
At such high temperatures, the atoms and molecules in the plasma gain enough energy to ionize, meaning they lose or gain electrons, resulting in a mixture of charged particles.
This ionization gives plasma its unique properties and behavior.
Plasma is commonly found in phenomena such as lightning, stars, and certain laboratory conditions. Its high temperature is necessary for sustaining the ionization and allowing the plasma to exhibit characteristics such as electrical conductivity and the ability to generate magnetic fields.
To know more about plasma, refer here:
https://brainly.com/question/31764858#
#SPJ11