Answers
Answer:
C.
Explanation:
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the mass of the soot, ashes, and gases, equals the original mass of the charcoal and the oxygen when it first reacted. So the mass of the product equals the mass of the reactant.
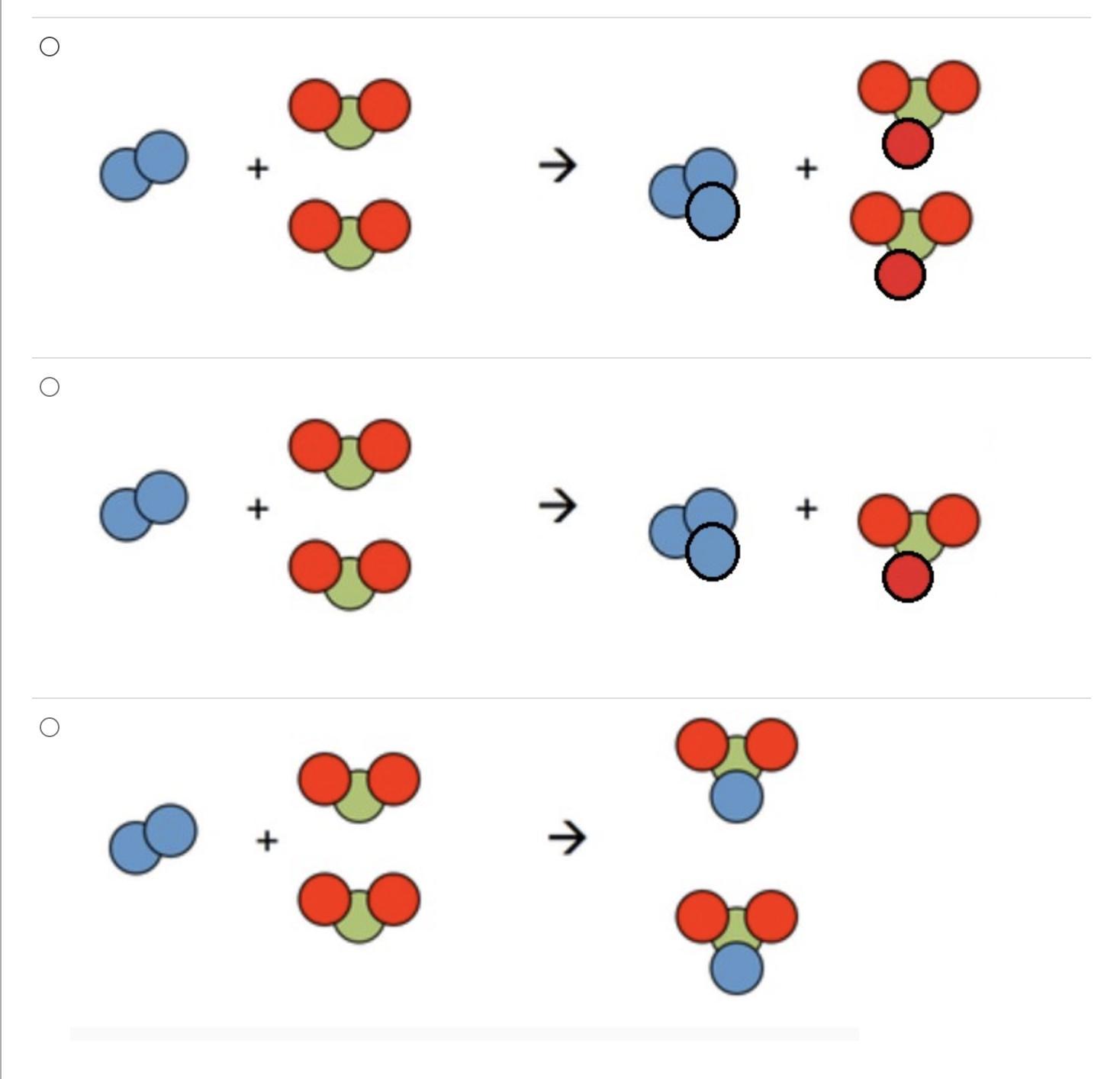
Answer:
C, since matter cannot be created or destroyed the same number of particles must be present on both sides of the equation. Hence why all chemical reactions must be balanced.
Explanation:
Related Questions
What is the atomic radius
of mercury?
Answers
The answer is 155 pm, hope this helps!
When naming a compound The first element is?
Answers
While naming a compound, the less electronegative element is written first in the formula.
Generally, the less electronegative element is written first while writing the formula, though there are a few exceptions. Basically, carbon is always written first in a formula and hydrogen is after nitrogen in a formula such as NH₃. The common order of common non-metals in binary compound formulas is C, P, N, H, S, I, Br, Cl, O, F.
Generally, electronegativity is defined as a chemical property which describes the tendency of an atom or a functional group to attract electrons toward itself. The electronegativity of an atom is basically affected by both its atomic number and the distance that its valence electrons reside from the charged nuclei. And the more electronegative element is written in the first name of the compound.
Learn more about electronegativity from the link given below.
https://brainly.com/question/17762711
#SPJ4
in a titration 20.0 mL of 0.150 M NaOH(aq) Solution exactly neutralize is 24.0 mL of an HCl solution what is the concentration of the HCl solution
Answers
Answer:
0.125
Explanation:
i took a test and got it right choosing this answer hopes this help
. How many ethyl groups are in the molecule 3, 3-diethylpentane?
O 1 groups
O 3 groups
O 4 groups
O2 groups
Answers
Answer:
2 groups
Explanation:
The molecule 3, 3-diethylpentane has 2 ethyl groups because the prefix di- means that there are 2 homogeneous substituent groups present in the molecule.
What is the theoretical yield of H 20 that can be obtained from the reaction of 4.5 g H 2 and excess O 2?
2H 2(g) + O2(g) → 2H 20(g)
4.5 g
40.8
80.g
9.0 g
81g
Answers
Answer:
9.0g
Whabshsbzhz sbsj dhbxjsbxjz sbsbbsbsjs
Explanation:
cause I believe
N2 + 3 H2 → 2 NH3
After performing an experiment, you find that when 52.5g
H2 reacts with excess N2, 290g NH3 is formed. What is the
percent yield of this reaction?
a. 90.3%
b. 98.2%
c. 89.1%
d. 102.4%
Answers
Answer:
B
Explanation:
what elements are always similar to each other on the period table?
Answers
The stoichiometry of the addition is 1:1, meaning that for every one mole of transcinnamic acid, one mole of Br2 is needed to form the addition product. The Br2 presents as a solution in dichloromethane solvent. This often causes problems for students when calculating stiochiometric equivalents. The bromine solution is 10% Br2 by volume. For instance, 100 mL of solution contains 10 mL of liquid Br2. The density of Br2 is 3.12 g/mL.
Calculate the number of mmol of Br2 present in 0.48 mL of bromine solution
Calculate the number of mmol in 100. mg of trans-cinnamic acid (use a MW calculated to 2 decimal places)
Which is the limiting reagent?
What is the theoretical yield of the addition product? (use a MW calculated to 2 decimal places)
Show all calculations.
Answers
The number of mmol of Br2 in 0.48 mL of bromine solution is 195 mmol. In 100 mg of trans-cinnamic acid, 0.674 mmol is calculated. The limiting reagent will be trans-cinnamic acid. The theoretical yield of the addition product is 211.9 mg.
To calculate the number of mmol of Br2 present in 0.48 mL of bromine solution, we need to first calculate the mass of Br2 in 0.48 mL of solution:
Volume of Br2 = 10% of 100 mL = 10 mL
Mass of Br2 = Volume x Density = 10 mL x 3.12 g/mL = 31.2 g
Now, we need to convert the mass of Br2 to mmol:
MM of Br2 = 159.8 g/mol
Moles of Br2 = Mass / MM = 31.2 g / 159.8 g/mol = 0.195 mol
Mmol of Br2 = Moles x 1000 = 195 mmol
Therefore, 0.48 mL of bromine solution contains 195 mmol of Br2.
To calculate the number of mmol in 100 mg of trans-cinnamic acid, we need to first calculate the molecular weight of trans-cinnamic acid:
MW of trans-cinnamic acid = 148.16 g/mol
Moles of trans-cinnamic acid = Mass / MW = 0.1 g / 148.16 g/mol = 0.000674 mol
Mmol of trans-cinnamic acid = Moles x 1000 = 0.674 mmol
Therefore, 100 mg of trans-cinnamic acid contains 0.674 mmol.
Since the stoichiometry of the reaction is 1:1, the limiting reagent will be the reactant with the lowest number of mmol. In this case, the limiting reagent will be trans-cinnamic acid since it only has 0.674 mmol, which is less than the 195 mmol of Br2.
To calculate the theoretical yield of the addition product, we need to use the number of mmol of the limiting reagent (0.674 mmol) and the molecular weight of the product:
MW of the addition product = 314.2 g/mol
Theoretical yield = Moles of limiting reagent x MW of product = 0.674 mmol x 314.2 g/mol = 211.9 mg
Therefore, the theoretical yield of the addition product is 211.9 mg.
Know more about trans-cinnamic acid here:
https://brainly.com/question/31656319
#SPJ11
I also really need help on this one.

Answers
Answer:
I would say ether the first one or the third one because on those questions you always want to choose the one that gives the most information or sound more believe
Hope this helped
Why do cells prefer purine and pyrimidine salvage pathways over the de novo synthesis pathway?
Answers
The cells prefer purine and pyrimidine salvage pathways over the de novo synthesis pathway because of their short act of enzymatic reactions.
What are salvage pathways?The salvage pathway is a set of acts or consequences of enzymatic or catalytic reactions to form the rapid products.
In the salvage pathway the enzymes which are used help purine and pyrimidine base pairs to transfer the electrons for metabolism.
Therefore, because of its short act of enzymatic reactions cells prefer purine and pyrimidine salvage pathways over the de novo synthesis pathway.
Learn more about salvage pathways, here;
https://brainly.com/question/28222638
#SPJ4
An infrared wave has a wavelength of 0.00005 m. What is this wavelength in
scientific notation?
A. 5.0 x 10-6 m
B. 5.0 x 10-5 m
C. 5.0 x 10-4 m
D. 5.0 x 10-3 m
Can someone please help me fast!!
Answers
Answer:
B. \( 5 \times {10}^{ - 5}\: m \)
Explanation:
\(0.00005 \: m= 5 \times {10}^{ - 5} \: m \\ \)
Can someone help me with this problem.

Answers
Answer:
c. a small amount
Explanation:
Trace elements (or trace metals) are minerals present in living tissues in small amounts.
Cations are
because?
Answers
Answer:
They are positively charged ions.
Explanation:
Though, I am not exactly sure about what do you actually mean by your question, but cations are positively charged ions.
2. A clear, colorless liquid is mixed with a clear, blue liquid resulting in a white powder suspended in a clear,
colorless liquid. Has a chemical reaction occurred? Why or why not?
Answers
Answer:
Yes, a chemical reaction has occurred
Explanation:
A chemical change is known to lead to the appearance of a new substance. Another name for chemical change is chemical reaction.
The new substance is formed when the bonds in the reactants are being rearranged to yield the products of the reaction.
In this case, two clear liquids were mixed and the contents of the system interacted briefly to yield a new product which appears as the white powder suspended in a clear, colorless liquid.
What happens to the atomic radius when an electron is lost?
A. The positive ionic radius is the same size as the neutral atomic
radius.
B. The positive ionic radius does not follow a trend with the neutral
radius.
C. The positive ionic radius is smaller than the neutral atomic radius.
D. The positive ionic radius is larger than the neutral atomic radius.
Answers
Answer:the positive ionic radius is smaller than the neutral atomic radius
Explanation:
Answer:The positive ionic radius is smaller than the neutral atomic radius.
Explanation: just took the test
Does acetone or n-hexane evaporate faster?
Answers
Answer:
acetone evaporates faster than hexane.
Explanation:
because acetone does nor participate in hydrogen bonding, so it's intermolecular forces are comparatively weaker, and it evaporates most quickly.
Find the molarity of a solution composed of 4.55 moles of kf in 0.75 lof solution.
Answers
The molarity (M) of a solution, you need to divide the moles of solute (in this case, KF) by the volume of the solution (in liters). The molarity of the solution is 6.07 M.
Molarity is defined as the number of moles of solute per liter of solution. In this problem, we are given the number of moles of solute (4.55 moles of KF) and the volume of solution (0.75 L). To find the molarity, we simply divide the number of moles by the volume in liters: Molarity = moles of solute / volume of solution. Molarity = 4.55 mol / 0.75 L, Molarity = 6.07 M.
Identify the moles of solute (KF): 4.55 moles
Identify the volume of the solution: 0.75 L
Divide the moles of solute by the volume of the solution: 4.55 moles / 0.75 L = 6.07 M.
To know more about solution visit:
https://brainly.com/question/15757469
#SPJ11
How would you synthesize the following compounds from butanoic acid using reagents from the table?
a) 2-bromobutane
b) 2-methylbutanoic acid
Answers
a) To synthesize 2-bromobutane from butanoic acid, react it first with thionyl chloride (SOCl2) and then with bromine (Br2) from the table.
b) To synthesize 2-methyl butanoic acid from butanoic acid, use a three-step process involving the reaction of butanoic acid with PCC to form butanal, then with methylmagnesium bromide (CH3MgBr) to form 2-methylbutan-1-ol, and finally with PCC again to form 2-methyl butanoic acid.
While the synthesis of 2-methyl butanoic acid requires a three-step process of oxidation, Grignard reaction, and oxidation, the synthesis of 2-bromobutane from butanoic acid only requires the conversion of the carboxylic acid to an acid chloride followed by a reaction with bromine.
Learn more about Grignard reaction
brainly.com/question/30546045
#SPJ4
how many moles are in 3.00x 10^22 atoms of He?
Answers
Answer:
0.05 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\\)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{3.0 \times {10}^{22} }{6.02 \times {10}^{23} } \\ = 0.049833...\)
We have the final answer as
0.05 molesHope this helps you
write the equation of the parabola that passes through the points (-12, 1333), (4,77), and (8,403)
Answers
To determine the equation of the parabola that passes through the given points, we can use the standard form of a quadratic equation, which is y = ax² + bx + c.
Substituting the coordinates of the three points into this equation will give us a system of three equations in three variables, which can be solved to find the values of a, b, and c that make up the equation of the parabola. The process is as follows:Substituting (-12, 1333) into the equation, we get: 1333 = a(-12)² + b(-12) + cSimplifying, we get: 1333 = 144a - 12b + c(1)Substituting (4,77) into the equation, we get: 77 = a(4)² + b(4) + cSimplifying, we get: 77 = 16a + 4b + c(2)Substituting (8,403) into the equation, we get: 403 = a(8)² + b(8) + cSimplifying, we get: 403 = 64a + 8b + c(3)Solving equations (1), (2), and (3) simultaneously, we get: a = -3, b = 45, and c = 1427Substituting these values back into the standard form equation, we get: y = -3x² + 45x + 1427Therefore, the equation of the parabola that passes through the points (-12, 1333), (4,77), and (8,403) is y = -3x² + 45x + 1427.
To know more about parabola visit :
https://brainly.com/question/11911877
#SPJ11
What volume is occupied by 3.00 moles of nitrogen under a pressure of 202 kPa and a temperature of 323K? R = 8.314 kPa L/mol K INICI
Answers
Answer:
39.9 L
Explanation:
Use PV = nRT to solve this problem.
Substitute the values known.
P is pressure, which is 202 kPa, V is volume, which we do not know, n is moles, which is 3, R is 8.314, and T is temperature, which is 323K.
Set up your equation as follows \(202 kPa V = 3.00mol (\frac{8.314 kPa L }{mol K} ) 323K\)
Solve for V, multiply the right side of the equation and divide by the pressure, 202 kPa.
This results in Volume being 39.9 L
What is the maximum mass of aluminum oxide that could be produced along with 48.96 g iron?
Answers
With 48.96 g of iron, the maximum mass of aluminum oxide that may be created is 22.3 g.
To determine the maximum mass of aluminum oxide that could be produced along with 48.96 g of iron, we need to first determine which reactant is limiting. The balanced chemical equation for the reaction between aluminum and iron (III) oxide is:
2Al(s) + Fe₂O₃(s) → 2Fe(s) + Al₂O₃(s)
From the equation, we can see that 2 moles of aluminum react with 1 mole of iron (III) oxide to produce 2 moles of iron and 1 mole of aluminum oxide.
We can use the molar mass of iron (III) oxide and the mass of iron provided to determine the number of moles of iron (III) oxide that reacted:
Molar mass of Fe₂O₃ = 55.85 g/mol + 2(16.00 g/mol)
= 159.69 g/mol
Number of moles of Fe = 48.96 g / 55.85 g/mol
= 0.876 mol
Number of moles of Fe₂O₃ = 0.876 mol / 2
= 0.438 mol
Therefore, 0.438 moles of iron (III) oxide reacted, which is the limiting reactant since there is less iron (III) oxide than required to react with all of the aluminum present.
Using the stoichiometry of the balanced equation, we can determine the maximum mass of aluminum oxide that can be produced:
1 mole of Al₂O₃ = 101.96 g
2 moles of Al react with 1 mole of Al₂O₃
Number of moles of Al₂O₃ = 0.438 mol / 2 = 0.219 mol
Mass of Al₂O₃ = 0.219 mol x 101.96 g/mol = 22.3 g
Therefore, the maximum mass of aluminum oxide that could be produced along with 48.96 g of iron is 22.3 g.
To know more about the Aluminum oxide, here
https://brainly.com/question/28544817
#SPJ1
For the water, what color is the hydrogen molecule?
Answers
Answer:
White is the color of a hydrogen molecule
Answer:
green. It is white when separate, but it turns green when it's formed into water.
Explanation:
balanced the equation please Fe + O2 → Fe2O3
Answers
Answer: The balanced equation is 4,3,2,
Answer: Balanced equation = 4Fe + 3O2 => 2Fe2O3.
Explanation:
for the following reaction, 0.270 moles of iron are mixed with 0.579 moles of oxygen gas
For the following reaction, 0.270 moles of iron are mixed with 0.579 moles of oxygen gas. iron(s) oxygen(g) → iron(II) oxide(s) What is the formula for the limiting reagent? What is the maximum amount of iron(II) oxide that can be produced?
Answers
The formula for the limiting reagent is Fe, and the maximum amount of iron(II) oxide that can be produced is 0.135 moles.
To determine the limiting reagent and the maximum amount of iron(II) oxide that can be produced, we need to compare the moles of each reactant and their stoichiometric ratios in the balanced equation.
The balanced equation for the reaction is:
4Fe(s) + 3O₂(g) → 2Fe₂O₃(s)
From the balanced equation, we can see that the stoichiometric ratio between iron and oxygen is 4:3. This means that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron(II) oxide.
Moles of iron(II) oxide = min (0.270 moles of Fe, (0.579 moles of O₂) × (2 moles of Fe₂O₃ / 3 moles of O₂))
To determine the limiting reagent, we compare the moles of iron and oxygen and choose the reactant that produces the lesser moles of iron(II) oxide. In this case, we have:
Moles of iron(II) oxide produced from 0.270 moles of Fe = 0.270 moles × (2 moles of Fe₂O₃ / 4 moles of Fe) = 0.135 moles
Moles of iron(II) oxide produced from 0.579 moles of O₂ = 0.579 moles × (2 moles of Fe₂O₃ / 3 moles of O₂) = 0.386 moles
Since 0.135 moles of iron(II) oxide is less than 0.386 moles, the limiting reagent is iron.
Therefore, the formula for the limiting reagent is Fe, and the maximum amount of iron(II) oxide that can be produced is 0.135 moles.
Learn more about limiting reagents here:
https://brainly.com/question/31171741
#SPJ11
Which of these ions is most likely to be leached from the soil?
a. magnesium ions,
b. chlorine ions,
c. calcium ions,
d. iron ions
e. potassium ions
Answers
what is the selenide ion concentration for a .200m h2s solution that has the stepwise dissociation constant
Answers
To determine the selenide ion (Se2-) concentration for a 0.200 M H2S solution that has the stepwise dissociation constant, we need to use the equilibrium constants for the reaction of H2S with water and for the reaction of HSe- with water.
The stepwise dissociation of H2S in water can be represented as:
H2S + H2O ⇌ HS- + H3O+
K1 = [HS-][H3O+] / [H2S]
The stepwise dissociation of HSe- in water can be represented as:
HSe- + H2O ⇌ Se2- + H3O+
K2 = [Se2-][H3O+] / [HSe-]
We are given that the concentration of H2S is 0.200 M. At equilibrium, some of the H2S reacts with water to form HS- and H3O+. We can assume that the initial concentration of H2S is much greater than the concentrations of HS- and H3O+ formed, so we can approximate the concentration of H2S to be 0.200 M at equilibrium. We don't know the concentration of H3O+ at this point, so we will express it in terms of x.
H2S + H2O ⇌ HS- + H3O+
Initial concentration: 0.200M 0 0 0
Change: -x +x +x
Equilibrium concentration: 0.200-x x x -
Now we can use the equilibrium concentrations to calculate the values of K1 and K2 using the given stepwise dissociation constants:
K1 = 1.1 × 10^-7 = [HS-][H3O+] / [H2S]
K2 = 1.3 × 10^-13 = [Se2-][H3O+] / [HSe-]
We can express [HSe-] in terms of [HS-] and [H2S] using the acid dissociation constant expression for H2S:
K1 = [HS-][H3O+] / [H2S]
1.1 × 10^-7 = x^2 / (0.200 - x)
Solving for x, we get:
x = 5.5 × 10^-5 M
This is the concentration of [HS-] and [H3O+] at equilibrium. To find the concentration of [Se2-], we can use the equilibrium constant expression for the reaction of HSe- with water:
K2 = [Se2-][H3O+] / [HSe-]
1.3 × 10^-13 = [Se2-](5.5 × 10^-5) / (0.200 - 5.5 × 10^-5)
Solving for [Se2-], we get:
[Se2-] = 2.6 × 10^-14 M
Therefore, the selenide ion concentration for a 0.200 M H2S solution that has the stepwise dissociation constant is 2.6 × 10^-14 M.
To know more about ion click here
brainly.com/question/14982375
#SPJ4
Jonathan conducts an experiment to determine what solutes readily dissolve in water. He places 3 tablespoons of potting soil into one cup of water. He records his observations in 15-minute increments. After a half hour, he notices that some of the soil particles have separated and sank to the bottom. O 15 minutes 30 minutes Which term best describes the combination of soil and water?
A.Mixture
B. A solution
C. an alloy
D. an emulsion
Answers
Answer:
A. Mixture
Explanation:
Why was there no reaction in some of the wells?
Answers
`Name:
Date:
Properties of Matter - Crunch time Review
1. If two objects balance like the ones shown below, what must be true?
A. Object A has more mass than object B.
Both objects have the same mass.
C. Object A has more volume than object B.
D. Both objects have the same volume.
Answers
Answer:
d
Explanation:
because i did it