Which of the following land features would most likely be formed at the boundary in the diagram?
Answers
Answer:
what diagram
Explanation:
Related Questions
Which chemical equation is unbalanced? c o2 right arrow. co2 sr o2 right arrow. 2sro 6h2 3o2 right arrow. 6h2o h2 h2 o2 right arrow. h2o h2o
Answers
The unbalanced equation is one in which the moles of atoms are not equal on both sides of the reaction.
What is a balanced chemical equation?A balanced chemical equation is one in wgich the moles of atoms in the reactants side is equal to the moles of atoms on the product side.
The given equations of reaction is not clearly stated.
Therefore, the unbalanced equation is one in which the moles of atoms are not equal on both sides of the reaction.
Learn more about balanced equations at: https://brainly.com/question/11904811
#SPJ4
According to a recent pol, 25% of adults in a certain area have high levels of cholesterol. They ceport that such elevated fevels "could be financialy devastating to the regions heathcare instem" and are a major concern to health insurance providers. Assume the standard deviation from the recent studies is accurate and known. According to recent studies, cholesterol levels in healthy adults from the area average about 205 mg/dL, with a standard deviation of about 35 mg/dL, and are roughly Normally distributed. If the cholesterol levels of a sample of 46 healthy adults from the region is taken, answer parts (a) through (d)
(a) What is the probability that the mean cholesterol level of the sample will be no more than 205?
Plys 205) 0.5 (Round to three decimal places as needed.)
(b) What is the probability that the mean cholesterol level of the sample will be between 200 and 2107
P(200
(c) What is the probability that the mean cholesterol level of the sample will be less than 1957
Ply<195) (Round to three decimal places as needed)
(d) What is the probability that the mean cholesterol level of the sample will be greater than 2179
Py>217) (Round to three decimal places as needed)
Answers
Hence, the probability that the mean cholesterol level of the sample will be greater than 217 is 0.034. Answer: 0.034.According to the given statement, we have the following data.
mean (μ) = 205 mg/dLstandard deviation
(σ) = 35 mg/dLsample size
(n) = 46(a) Probability that the mean cholesterol level of the sample will be no more than 205.To find this, we will use the z-score formula.z
= (x - μ) / (σ/√n)Here,
x = 205
μ = 205
σ =
35n
= 46Plugging in these values, we get,
z = (205 - 205) / (35/√46)
z = 0Hence, the probability that the mean cholesterol level of the sample will be no more than 205 is 0.5. Answer: 0.5
(b) Probability that the mean cholesterol level of the sample will be between 200 and 210:
To find this, we need to standardize the values and use the z-table.P(z < (210 - 205) / (35/√46)) - P(z < (200 - 205) / (35/√46))P(z < 1.65) - P(z < -1.65) = 0.4495 - 0.0505
= 0.3990Hence, the probability that the mean cholesterol level of the sample will be between 200 and 210 is 0.3990. Answer: 0.3990
(c) Probability that the mean cholesterol level of the sample will be less than 195: To find this, we need to standardize the values and use the z-table.P(z < (195 - 205) / (35/√46))P(z < -2.91) = 0.002Hence, the probability that the mean cholesterol level of the sample will be less than 195 is 0.002. Answer: 0.002
(d) Probability that the mean cholesterol level of the sample will be greater than 217: To find this, we need to standardize the values and use the z-table.P(z > (217 - 205) / (35/√46))P(z > 1.82) = 0.034 Answer: 0.034.
To know more about data visit:
https://brainly.com/question/29117029
#SPJ11
say hiii for the camera
Answers
Answer:
Hello?
Explanation:
Answer:
hiiiiii camreaaaaaa. also hows youre dayy
Explanation:
hi :]
a) the use of heat energy to react carbon dioxide with water to produce oxygen. b) a chemical reaction between carbohydrates and oxygen to produce carbon dioxide, water, and heat energy. c) a photochemical reaction involving carbon dioxide and water to produce carbohydrates and oxygen. d) a photochemical reaction involving carbon dioxide and oxygen to produce water and oxygen.
Answers
Answer: b) a chemical reaction between carbohydrates and oxygen to produce carbon dioxide, water, and heat energy
Explanation:
Based on the options, I assume that this is talking about the process of respiration. Respiration allows living things to produce energy by reacting carbohydrates with oxygen.
This results in the release of energy in the form of heat in a cell and also the formation of carbon dioxide and water. This type of respiration is known as Aerobic respiration.
heterogeneous non example
Answers
Heterogeneous 'mixtures' (because they don't meet the definition of mixtures) are mixtures substances that aren't completely uniformly spread out. They haven't reacted with the solvent to become a solution.
Explanation: There are 2 types of heterogeneous solutions, A Colloid and a suspension.
Colloid: You can check if a mixture is heterogeneous by passing a light ray through it. This may cause the Tyndall effect (If the mixture is a colloid) when the Colloidal Heterogeneous mixture's particles are so small that they refract the beam of light and the path of light will be visible, like if you add 3 drops of milk in a glass of Water and shine a laser light through it. This is because the particles are too small to be seen by the unaided eye but big enough to scatter you laser light. However that particles won't settle down or will be separated by a filter paper due to particles' small size.
Suspension: A solution will be a solution when the particles of the Mixture is big enough to be seen by the unaided eye. Like if you mix sand and Water, the sand will eventually settle down due to Gravity. The mixture's solute will be big enough to pass through a filter paper.
Which of the following is the most stable isotope? Will give BRAINLIEST!!
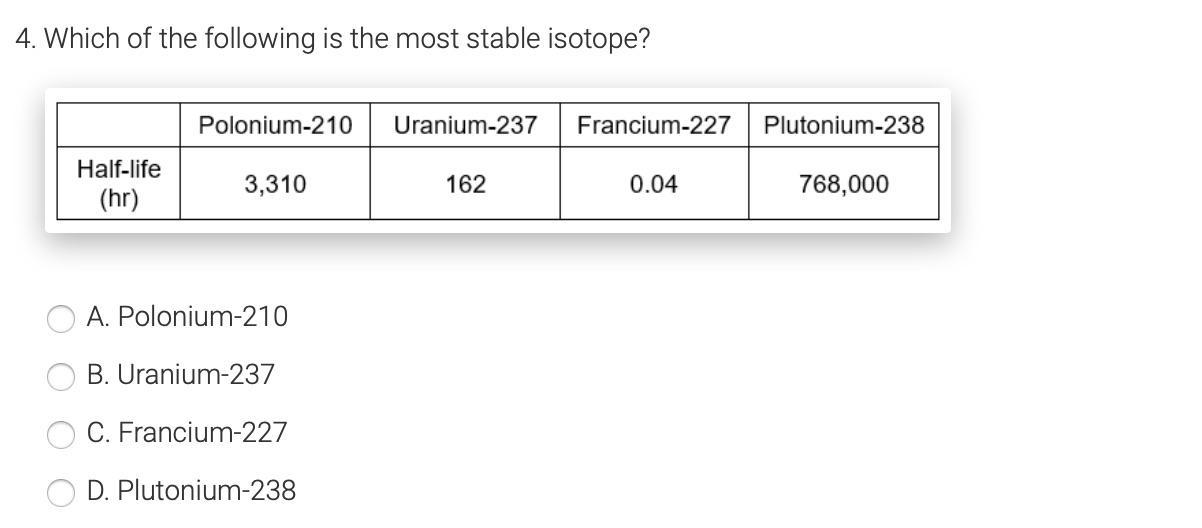
Answers
Answer:
D
Explanation:
Because it has the highest number
A THE ANSWER IS A
U WELCONM
assign oxidation numbers to each of the atoms br, o in bro−3.
Answers
The oxidation numbers of the atoms in the BrO3- ion are:
Bromine (Br): +6
Oxygen (O): -2 (three times)
What is the oxidation number?In the Bro3- ion, the total charge of the ion is -1.
To assign oxidation numbers, we start with the general rule that the oxidation number of oxygen is almost always -2, and the oxidation number of hydrogen is +1. The oxidation number of fluorine is always -1, and the oxidation number of other halogens, like bromine, is usually -1 but can vary depending on the compound.
Therefore, we can assign the oxidation number of -2 to each of the three oxygen atoms in the BrO3- ion.
Let x be the oxidation number of bromine in the BrO3- ion.
Using the fact that the total charge of the ion is -1, we can write the following equation:
-2 × 3 + x + (-1) = -1
Simplifying this equation, we get:
-6 + x - 1 = -1
x - 7 = -1
x = +6
So, the oxidation number of bromine in the BrO3- ion is +6.
Therefore, the oxidation numbers of the atoms in the BrO3- ion are:
Bromine (Br): +6
Oxygen (O): -2 (three times)
Learn more about oxidation numbers
brainly.com/question/29100691
#SPJ11
What type of Star is this?

Answers
Answer:
rock star
Explanation:
It takes 2,500,00 Liters of Helium to fill the Goodyear Blimp. How many moles is this?
Answers
Answer:
102.26 moles of helium were required to Fill the Goodyear Blimp
Explanation:
To solve this question we need to use combined gas law:
PV = nRT
Where P is pressure, V is volume of gas (2500L), n are moles of gas (Our incognite), R is gas constant (0.082atmL/molK) and T is absolute temperature
Assuming atmospheric condition we can write P = 1atm and T = 25°C = 298.15K
Replacing:
PV/RT = n
1atm*2500L / 0.082atmL/molK*298.15K = n
102.26 moles of helium were required to Fill the Goodyear Blimp
What do you suspect would have happened if 10.0 ml of ethyl acetate were used as the reaction solvent as opposed to a mixture of 5.0 ml ethyl acetate and 5.0 ml of hexane?
Answers
It could potentially enhance or inhibit the reaction depending on the specific reaction conditions.
The use of different solvents can affect the rate and efficiency of a reaction. Ethyl acetate and hexane have different polarity levels, with ethyl acetate being more polar than hexane. This difference in polarity can impact the solubility and interactions between the reactants and other compounds involved in the reaction.
If 10.0 ml of ethyl acetate were used as the reaction solvent, the increased polarity of ethyl acetate may affect the solubility and reactivity of the reactants.
To know more about reactants visit:-
https://brainly.com/question/14449229
#SPJ11
5. Identify the sums or differences of the following:
1)
(8.41 X 104) + (9.71 X 104) =
2)
(5.11 X 102) - (4.2 X 102) =
3)
(8.2 X 103) + (4.0 X 103) =
4)
4
(6.3 X 10 9 - (2.1 X 102) =
Answers
I will do my best to help! Since I'm not the best at explaining things, I will just show my work. For number four, I wasn't exactly sure what you meant so I just solved it in different ways depending on the different ways I thought you meant. I'm sorry if I didn't end up solving it the way you wanted though. Either way, I really hope I helped you out!
1.
(8.41 X 104) + (9.71 X 104)
874.64 + 1009.84
= 1884.48
2.
(5.11 X 102) - (4.2 X 102)
521.22 - 428.4
= 92.82
3.
(8.2 X 103) + (4.0 X 103)
844.6 + 412
= 1256.6
4. (If the equation was supposed to be "(6.3 X 10^9) - (2.1 X 102)")
(6.3 X 10^9) - (2.1 X 102)
(6.3 x 1000000000) - (2.1 X 102)
6300000000 - 214.2
= 6299999785.8
4. (If the equation was supposed to be "(6.3 X 109) - (2.1 X 102)")
(6.3 X 109) - (2.1 X 102)
686.7 - 214.2
= 472.5
Both the sum and difference can be used in various contexts, such as solving equations, calculating measurements, or analyzing data. These operations are fundamental in mathematics and are often used in everyday situations where numbers need to be combined or compared.
1) (8.41 x 10⁴) + (9.71 x 10⁴) = 1.521 x 10⁵
To find the sum, add the numbers in scientific notation by ensuring that the exponents are the same. In this case, since both numbers have an exponent of 4, you can add the coefficients: 8.41 + 9.71 = 18.12. The result is then expressed in scientific notation as 1.812 x 10⁵, which is equivalent to 1.521 x 10⁵ after rounding to three significant figures.
2) (5.11 x 10²) - (4.2 x 10²) = 0.91 x 10²
To find the difference, subtract the numbers in scientific notation while keeping the exponents the same. In this case, both numbers have an exponent of 2. Subtracting the coefficients gives you: 5.11 - 4.2 = 0.91. The result is then expressed in scientific notation as 9.1 x 10¹, which is equivalent to 0.91 x 10² after rounding to two significant figures.
3) (8.2 x 10³) + (4.0 x 10³) = 12.2 x 10³
To find the sum, add the numbers in scientific notation by ensuring that the exponents are the same. In this case, both numbers have an exponent of 3. Adding the coefficients gives you: 8.2 + 4.0 = 12.2. The result is then expressed in scientific notation as 1.22 x 10⁴ after rounding to three significant figures.
4) (6.3 x 10⁹) - (2.1 x 10²) = 6.3 x 10⁹
To find the difference, subtract the numbers in scientific notation while keeping the exponents the same. In this case, the exponents are different, but when subtracting a small value like (2.1 x 10²) from a large value like (6.3 x 10⁹), the smaller value becomes insignificant. Therefore, the result is approximately equal to the larger value: 6.3 x 10⁹.
Learn more about Exponent here:
https://brainly.com/question/13669161
#SPJ3
Determine the carburizing time necessary to achieve a carbon concentration of 0. 30 wt% at a position 4 mm into an iron–carbon alloy that initially contains 0. 10 wt% C. The surface concentration is to be maintained at 0. 90 wt% C, and the treatment is to be conducted at 1100°C. Use the diffusion data for γ-Fe in Table 5. 2. ( Callister, Materials Science and Engineering, 9th ed. , John Wiley & Sons, Inc. , 2014) Express your answer in hours to three significant figures
Answers
The carburizing time necessary to achieve a carbon concentration of 0.30 wt% at a position 4 mm into an iron-carbon alloy is 63.4 hours.
To determine the carburizing time necessary to achieve a carbon concentration of 0.30 wt% at a position 4 mm into an iron-carbon alloy, we can use Fick's second law of diffusion:
\(DC_{surface} / 2 = (C_{surface} - C_{4mm}) / erf(x / (2 * \sqrt{Dt} ))\\\)
where D is the diffusion coefficient, \(C{surface}\\\) is the surface carbon concentration (0.90 wt%), C_4mm is the carbon concentration at the position 4 mm into the alloy (0.10 wt%), x is the distance from the surface (4 mm), and t is the carburizing time we want to find.
We can use the diffusion coefficient for γ-Fe at 1100°C from Table 5.2, which is D = \(6.0 * 10^{-12} m^2/s.\)
Substituting the given values, we get:
\((6.0 * 10^{-12} m^2/s) * (0.90 - 0.30) / 2 = (0.90 - 0.10) / erf(4 mm / (2 * \sqrt{6.0 * 10^{-12} m^2/s} ))\)
Simplifying the left-hand side, we get:
\(1.8 * 10^{-12} m^2/s = (0.80) / erf(4 mm / (2 * \sqrt{(6.0 * 10^{-12} m^2/s) * t)})))\)
Taking the inverse error function of both sides, we get:
\(erf(4 mm / (2 * \sqrt{6.0 * 10^{-12} m^2/s) * t)} ) = 0.000346\)
Substituting this back into the previous equation, we get:
\(1.8 * 10^{-12} m^2/s = (0.80) / 0.000346\)
Solving for t, we get:
t = 63.4 hours
For more question on diffusion click on
https://brainly.com/question/30900484
#SPJ11
When sulfur-35 (Z=16) decays to chlorine-35 (Z=17) a particle
emitted is_____
a) an alpha particle
b) A beta particle
c) A gamma ray
d) an x-ray
e) None of the above
Answers
When sulfur-35 (Z=16) decays to chlorine-35 (Z=17) a particle emitted is a beta particle. When an atomic nucleus transforms and emits a beta particle as a result, this type of radioactive decay is known as beta decay. Hence option B is correct.
Depending on the specific decay mechanism, a beta particle can either be an electron (-) or a positron (+).
A beta particle is released when chlorine-35 decays to sulfur-35. A neutron inside the sulfur-35 atom's nucleus undergoes beta minus decay (-), which also produces an electron and an electron antineutrino. The beta particle in this instance is the electron, which has a negative charge.
To know more about chlorine:
https://brainly.com/question/19460448
#SPJ4
The correct answer is B
When sulfur-35 (Z=16) decays to chlorine-35 (Z=17), a particle emitted is a beta particle.
Sulfur-35 decays to Chlorine-35 by a beta emission process. In beta emission, a neutron is converted into a proton and an electron. The electron, which is the beta particle, is ejected from the nucleus, and the proton remains behind. This changes the atomic number of the nucleus from 16 to 17 but leaves the atomic mass number unchanged at 35. Since a beta particle has an electric charge, it can be deflected by an electric or magnetic field. It is, therefore, easier to detect than a neutron or a gamma ray. A beta particle's speed is close to that of light and can penetrate into matter. However, it is easily stopped by a thin layer of metal or plastic. A beta particle's symbol is β-.
Learn more about beta emission process
https://brainly.com/question/30025290
#SPJ11
5. (30 points) The oil and water relative permeabilities for a chalk core plug are expressed by the following equations:
k
rw
=0.52(S
w
−0.25)
3
k
ro
=3.62(0.75−S
w
)
3
Determine the values of irreducible water saturation, residual oil saturation, and end-point relative permeabilities to oil and water.
Answers
The values of irreducible water saturation, residual oil saturation, and end-point relative permeabilities to oil and water for the chalk core plug are:
Irreducible water saturation (Swi) = 0.25 Residual oil saturation (Sor) = 0.75 End-point relative permeability to water (krw) = 0 End-point relative permeability to oil (kro) = 0In the given equations, the relative permeabilities for oil (kro) and water (krw) are expressed as functions of water saturation (Sw). To determine the values of irreducible water saturation (Swi), residual oil saturation (Sor), and end-point relative permeabilities, we need to analyze the equations.
From the equation for krw, we can observe that when Sw = Swi, krw = 0. Therefore, the irreducible water saturation (Swi) is 0.25.
From the equation for kro, we can see that when Sw = 1 (100% water saturation), kro = 0. This indicates that at maximum water saturation, there is no flow of oil, and the end-point relative permeability to oil (kro) is 0.
The end-point relative permeability to water (krw) can be determined by substituting Sw = 1 in the equation for krw. This gives us krw = 0.52\((1 - 0.25)^3\) = 0.199. Therefore, the end-point relative permeability to water is 0.199.
The residual oil saturation (Sor) can be calculated by substituting Sw = 0 in the equation for kro. This gives us kro = 3.62 \((0.75 - 0)^3\) = 3.245. Therefore, the residual oil saturation is 0.75.
Learn more about Relative permeability
brainly.com/question/28900728
#SPJ11
A graduated cylinder contains 20.0 mL of water. After a rock is placed in the cylinder, the level rises as shown below. What is the volume of the rock?
Answers
Answer:
The correct answer is 14 cm³.
Explanation:
Based on Archimedes Principle at rest when a body is completely or partially immersed within a fluid, it encounters a vertical thrust, which is equivalent to the weight of the fluid that the body displaces. In the mentioned case, the fluid is water. Thus, the rock being denser will result in the displacement of water, making the level of water to ascend in the cylinder.
Thus, if V₁ or the initial volume is 20 ml or 20 cm³, and after insertion of the rock, the rise in the level of water becomes V₂ as 34 ml or 34 cm³. Then the volume of the rock will be,
V = V₂ -V₁ = 34 - 20 = 14 cm³.
Modifying the game for less skilled players includes all of the following EXCEPT:
A
giving an extra chance to players who struggle
B
rotating teams more frequently
c
selecting teams based on height
d
altering the rules of the game to accommodate
Answers
Answer:
c
Explanation:
took the exam
Is carbon dioxide a polar molecule? Why or Why Not?
Answers
Explanation:
Carbon dioxide isn't a polar molecule because
same nature of charge appears in opposite direction which cancel each other. So, carbon dioxide is non-polar molecule.
a certain substance has a heat of vaporization of 26.64 kj/mol.26.64 kj/mol. at what kelvin temperature will the vapor pressure be 8.008.00 times higher than it was at 287 k?
Answers
At 457.3 K temperature, the vapour pressure will be higher, for the given substance and its heat of vapourization.
What is Vapour pressure?The pressure that a vapour exerts on its condensed phases in a closed system when they are in thermodynamic equilibrium with one another at a specific temperature is known as vapour pressure. A liquid's evaporation rate can be determined by looking at the equilibrium vapour pressure.
Calculation:
The Clausius-Clapeyron equation is as follows:
ln (P2/P1) = ∆Hvap/R * (1/T1 - 1/T2)
ln (8.008) = (26.64 * 10³J/mol) / (8.314J/mol/K) * {(1/287K) - (1/T2) }
2 = (3.2 * 10³/K) * {(T2 - 287K)/ (287T2 * K)
0.000625/K * 287T2 * K = T2 - 287K
179.375K = T2 -287K
T2 = 457.3K.
Hence, at 457.3 K temperature, the vapour pressure will be higher.
To know more about Vapour pressure, check out:
https://brainly.com/question/2272852
#SPJ4
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
Equilibrium calculations: Using the equation below for calculating the equilibrium constant for the reaction of PCl3 + Cl2 to produce PCs, make the following substitutions for the reactants and products, and recalculate the equilibrium constant, then comment on the equilibrium constant does it favor products, or reactants? [PCI) = 5.0 mol/L [Cl2] = 5.0 mol/L [PC1s] = 0.1 mol/L [PCI ] 0.050 mol/L K= 9.6 x 10-4 [PC13 ][CI] (7.2 mol/L) (7.2 mol/L) For the steam reformation reaction CH + H2O + CO + 3 H2, calculate the equilibrium constant using the formula and concentrations below, and comment on the equilibrium constant: does it favor products, or reactants? What do you think is the industrial use of this chemical reaction?
Answers
The equilibrium constant is 12.5
The equilibrium constant of a chemical response is the price of its response quotient at chemical equilibrium, a kingdom approached by means of a dynamic chemical device after enough time has elapsed at which its composition has no measurable tendency towards in addition trade.The equilibrium consistent of a chemical response is the price of its response quotient at chemical equilibrium, a kingdom approached by a dynamic chemical device after enough time has elapsed at which its composition has no measurable tendency in the direction of further trade.
Calculations:-
CH₄ + H₂O ---------> CO + 2H₂
[CO] = 10⁻² mol/L
= 0.01 mol/l
[H₂] = 5 × 10⁻¹ mol/l = 0.5 mol/l
[H₂O] = 10⁻² mol/l = 0.01 mol/l
[CH₄] = 10⁻² mol/l = 0.01 mol/l
K = [CO][H₂]³/[CH₄][H₂O]
= (0.01) × (0.5)³/(0.01)×(0.01)
Kc = 12.5
Equillibirium constant = 12.5
To lean more about equilibrium constant :
brainly.com/question/3159758
#SPJ4
Write the rate law for the following elementary reaction: ICI(g) + H2(g) → HI(g) + HCl (g) Use k, to stand for the rate constant. rate =
Answers
The rate law for an elementary reaction is determined solely by the stoichiometry of the reactants. Since the reaction given is elementary, we can determine the rate law by looking at the coefficients of the reactants. In this case, we see that the rate of the reaction is directly proportional to the concentrations of ICI and H2. Therefore, the rate law for the reaction is rate = k[ICI][H2].
The rate constant, k, is a proportionality constant that depends on the temperature, the presence of catalysts, and other factors. To write the rate law for the given elementary reaction: ICl(g) + H2(g) → HI(g) + HCl(g), we need to consider the rate constant (k) and the concentrations of the reactants. The rate law for this reaction is given by:
Rate = k [ICl] [H2]
In this equation, "k" is the rate constant, "[ICl]" represents the concentration of ICl, and "[H2]" represents the concentration of H2. The rate law shows that the rate of the reaction is directly proportional to the product of the concentrations of the reactants, ICl and H2.
To know more about rate constant visit-
https://brainly.com/question/20305871
#SPJ11
If 7.34 mol of O2 reacts, calculate the grams of CO2 produced.CH4 + 2O2—> CO2 + 2H2O
Answers
Answer:
\(161.48\text{ g}\)Explanation:
Here, we want to get the mass of carbon (iv) oxide produced
From the question, we have the balanced chemical reaction stating that 2 moles of oxygen molecule produced 1 mole of carbon (iv) oxide molecule
The number of moles of carbon (iv) oxide produced from 7.34 mol oxygen is thus:
\(\frac{7.34\times1}{2}\text{ = 3.67 moles}\)1 mole of carbon (iv) oxide contains 44 g
The mass in 3.67 moles will be:
\(44\times3.67\text{= 161.48 g}\)TRUE OR FALSE
All of the elements on Earth, except for hydrogen, were formed in the interiors of stars.
Answers
Answer:
True
Explanation:
I checked several websites to double check for you.
Answer:
Except for hydrogen and some helium created in the Big Bang, all of the stuff we, and the Earth around us, are made of, was generated in stars, through sustained fusion or in supernova explosions i thank its true :) hope this helps
The pressure and temperature of N² and gas drops from 303kPa at 300K to 202²kPa at -73°C. If the initial volume is 2L, find the new volume.
Answers
Answer:
Gjvb
Explanation:
Using the combined gas law equation as follows:
P1V1/T1 = P2V2/T2
Where;
P1 = initial pressure (kPa)
P2 = final pressure (kPa)
V1 = initial volume (L)
V2 = final volume (L)
T1 = initial temperature (K)
T2 = final temperature (K)
According to this question;
T1 = 300K
T2 = -73 + 273 = 200K
P1 = 303kPa
P2 = 202kPa
V1 = 2L
V2 = ?
Using the above formula:
303 × 2/300 = 202 × V2/200
606/300 = 202V2/200
Cross multiply;
606 × 200 = 300 × 202V2
121200 = 60600V2
V2 = 121200/60600
V2 = 2L
The new volume is 2L
give the iupac name for this molecule. this molecule has the condensed formula c h 3 c h 2 c h (c h 3) c h 2 c h 2 c h o.
Answers
The IUPAC name for this molecule will be 4-methyl hexaldehyde.
An worldwide federation of National Adhering Organizations seeking to improve the chemical sciences, particularly by creating nomenclature and vocabulary, is the International Union of Pure and Applied Chemistry. The International Science Council counts it among its members.
Whether in a continuous chain or a ring, the longest chain of carbons joined by a single bond serves as the basis for IUPAC nomenclature. According to a particular set of priorities, all deviations, whether multiple bonds or atoms other than carbon and hydrogen, are denoted by prefixes or suffixes.
For this molecule we know that if aldehyde group is present then the numbering will start from there and one methyl group is present on 4th carbon thus the IUPAC name will be 4-methyl hexaldehyde.
To know more about IUPAC visit the link:
https://brainly.com/question/16631447?referrer=searchResults
#SPJ4
importance of mole ratio in solvey process
Answers
Answer:
Sry i accidently clicked on ''SAVE''.
U can remove it or report it...
Explanation:
How many formula units of CaF2 are in 15 grams of CaF2
Answers
15 grams of CaF2 is equal to 0.844 moles. Therefore, there are 0.844 moles of CaF2, or 6.752 formula units of CaF2.
WILL MARK BRAINLIST IF RIGHT
What coefficient should be used for oxygen gas to correctly balance this equation

Answers
Answer:
4FeS +3O2-2Fe2O3+4SO2
according to rutherford's nuclear theory, the number of negatively charged particles outside the nucleus is blank the number of positively charged particles within the nucleus, so a nitrogen atom has 7 protons and 7 electrons, while a phosphorous atom cannot have 15 protons and 150 electrons.
Answers
According to Rutherford's nuclear theory, the number of negatively charged particles outside the nucleus is equal to the number of positively charged particles within the nucleus.
Rutherford's nuclear hypothesis states that ratio of negatively charged particles outside nucleus to positively charged particles inside the nucleus is one-to-one. This implies that the number of protons and electrons in an atom is equal. An atom with an atomic number of 7 has 7 protons and 7 electrons, which suggests it is a nitrogen atom.
The negative charge of electrons in an electron cloud encircling the nucleus balances the positive charge of the protons in the nucleus to produce a neutral atom. However, the periodic table indicates that phosphorus has an atomic number of 15, which corresponds to a total of 15 protons. There would be 15 electrons in a neutral phosphorus atom to counteract the protons' positive charge.
Read more about nuclear theory on:
https://brainly.com/question/28500137
#SPJ4
a vessel contains a stoichiometric mixture of butane and air. the vessel is at a temperature of 500 k, a pressure of 1 atm, and has a volume of
Answers
The final pressure and temperature are 1.131 atm and (0.9786 mol/ 0.8546 mol).
What is a chemical equation with an example?A chemical equation serves as a metaphor for the transformation of reactants into products. Iron sulfide, for instance, is created when iron (Fe) and sulfur (S) mix (FeS). Fe(s) + S(s) = FeS (s) Iron reacts with sulfur, as indicated by the + sign.
For the complete combustion of butane, the following chemical equation is balanced:
2C4H10 + 13O2 → 8CO2 + 10H2O
mass of butane = (number of moles of butane) x (molar mass of butane)
= (number of moles of oxygen) x (molar mass of oxygen)
= (mass of oxygen) / (molar mass of oxygen) x (molar mass of butane)
The mass of oxygen can be calculated from the ideal gas law:
PV = nRT
n = PV / RT
The amount of moles of oxygen can be determined using this equation with P = 1 atm, V = 5 L, and T = 500 K:
n = (1 atm) x (5 L) / [(0.08206 L atm mol⁻¹ K⁻¹) x (500 K)]
= 0.1222 mol
The mass of butane is:
mass of butane = (0.1222 mol) x (58.12 g/mol)
= 7.11 g
Before the reaction, there were n = 0.1222 mol (butane) + (13/2) x 0.1222 mol moles of gas in the vessel (oxygen)
= 0.8546 mol
The balanced equation:
n = (8/2) x 0.1222 mol (carbon dioxide) + (10/2) x 0.1222 mol (water vapor)
= 0.9786 mol
Solving for P2, we get:
P2 = (n2 / n1) x (T1 / T2) x P1
= (0.9786 mol / 0.8546 mol) x (500 K / T2) x (1 atm)
= 1.131 atm
Solving for T2, we get:
T2 = (n2 / n1) x (P1 / P2) x T1
= (0.9786 mol / 0.8546 mol)
To know more about final pressure visit:-
https://brainly.com/question/30862968
#SPJ1
Question:
A vessel contains a stoichiometric mixture of butane and air. The vessel is at a temperature of 500 K, a pressure of 1 atm, and has a volume of 5 L. If the reaction goes to completion, what volume of gas will be present in the vessel after the reaction and what will be the final pressure and temperature? Assume ideal gas behavior and that the reaction occurs with complete combustion.