Answers
The ratio between N2 and the product is 1:2.
The ratio between H2 and the product is 3:2.
These ratios are deducted from the stoichiometry coefficients, which indicate the molar quantity of each molecule.
From these ratios, we can deduct the answer most contain greater moles for H2 than for N2 because the hydrogen molecule represents more product, this leaves us with two possible options: a and b.
At last, if we use 2 moles of N2 and 4 moles of H2, we would get the greatest amount of product, and we are using the proper ratio indicated by the chemical equation.
Therefore, the answer is a.
Related Questions
WILL MARK BRAINLEST
what two factors determine the absolute brightness of a star
Answers
Answer:
the brightness of a star depends on its composition and how far it is from the planet
Calculate and record the following data in the table. Name Propane Butane Methane
Molar volume (L/mol) _____ _____ _____
Average molar volume (L/mol) _______________________
Propane molar volume: ______ L/mol butane molar volume: ______ L/mol
Answers
The molar volume and average molar volume of propane, butane, and methane at STP are 24.45 L/mol , 24.93 L/mol ; 28.02 L/mol , 24.93 L/mol and 22.41 L/mol , 24.93 L/mol respectively.
The molar volume of a gas is defined as the volume occupied by one mole of gas at standard temperature and pressure (STP), which is defined as 0°C and 1 atm.
The molar volume of a gas can be calculated using the ideal gas law,
PV = nRT,
where P is pressure, V is volume, n is number of moles, R is the gas constant, and T is temperature in kelvins.The molar volumes of propane, butane, and methane at STP can be calculated as follows:
Propane: C₃H₈
At STP (0°C and 1 atm), the molar volume of propane can be calculated as:
V = (nRT) / P
V = (n x 0.0821 x (273.15 + 0) / 1) / 1
V = 24.45 L/mol
Butane: C₄H₁₀
At STP (0°C and 1 atm), the molar volume of butane can be calculated as:
V = (nRT) / P
V = (n x 0.0821 x (273.15 + 0) / 1) / 1
V = 28.02 L/mol
Methane: CH₄
At STP (0°C and 1 atm), the molar volume of methane can be calculated as:
V = (nRT) / P
V = (n x 0.0821 x (273.15 + 0) / 1) / 1
V = 22.41 L/mol
The average molar volume is the sum of the molar volumes divided by the number of gases:
Average molar volume = (24.45 + 28.02 + 22.41) / 3
Average molar volume = 24.93 L/mol
Learn more about molar volume at :https://brainly.com/question/29884686
#SPJ4

A teacher showed this animal to studenst on a field trip
Answers
If a teacher showed an animal to students on a field trip. The tool will allow the students to best see the animal up close is the hand lens.
Option D is correct.
What is a Hand lens?A hand lens is known as a magnifying glass which is a convex lens that is used to produce a magnified image of an object. The lens is usually mounted in a frame with a handle.
A hand lens has two essential properties which are its focal length and its diameter.
The students will therefore require a hand lens to look up the animal close.
Learn more about hand lens at:https://brainly.com/question/12027484
#SPJ1
#complete question:
A teacher showed this animal to students on a field trip. Which tool will allow the students to best see the animal up close? O A Tape measure O B Graduated cylinder O c. Notebook O D. Hand lens
Determine the quantity in moles of RbF that are in 57.0 grams of RbF.
Answers
The quantity of moles in 57.0grams of RbF is 0.545mol.
How to calculate number of moles?Moles is the base unit of amount of substance i.e. the amount of substance of a system which contains exactly 6.022 × 10²³ elementary entities (atoms, ions, molecules, etc.).
The number of moles of a substance can be calculated by dividing the mass of the substance by its molar mass.
No of moles = mass ÷ molar mass
Molar mass of RbF = 85.467 + 18.998 = 104.5g/mol
moles = 57.0g ÷ 104.5g/mol = 0.545mol
Therefore, 0.545moles is the quantity of moles of RbF in 57.0grams of the compound.
Learn more about no of moles at: https://brainly.com/question/12513822
#SPJ1
(a) Thomson’s cathode–ray tube (Figure 2.6) and the mass spectrometer (Figure 2.15) both involve the use of electric or magnetic fields to deflect charged particles. What are the charged particles involved in each of these experiments?
Answers
The charged particles involved in Thomson’s cathode–ray tube experiment is the negatively charged particles that we now call electrons.
The charged particles involved in the mass spectrometer is positively charged ions that is being deflected by magnetic fields.
What is the mass spectrometer?The Mass spectrometer is an analytical tool that is used to measure the mass-to-charge ratio of ions.
One of the advantages of the mass spectrometer is that it is an excellent tool for identifying unknown components in a sample or confirming their presence.
The major disadvantages of mass spectrometer is that it is not very good at identifying hydrocarbons that produce similar ions and it's unable to tell optical and geometrical isomers apart.
In conclusion, some applications of mass spectrometer includes drug testing and discovery, food contamination detection.
Learn more about mass spectrometer at: https://brainly.com/question/28174174
#SPJ1
An antibiotic solution must be prepared by diluting 100. mL of a 90. % m/v solution and bringing up the volume to 250 mL. What is the concentration of the resulting solution?
Answers
Answer:
36% m/v
Explanation:
An antibiotic solution must be prepared by diluting 100. mL of a 90. % m/v solution and bringing up the volume to 250 mL. What is the concentration of the resulting solution?
the new solution is 100/250 X 90% m/v = 36% m/v
The concentration of the resulting antibiotics solution that must be prepared by diluting 100mL of a 90. % m/v solution and bringing up the volume to 250 mL is 36% m/v.
How to calculate concentration?The concentration of a solution can be calculated by using the following formula:
C1V1 = C2V2
Where;
C1 = initial concentrationC2 = final concentrationV1 = initial volumeV2 = final volume90% m/v × 100 = C2 × 250
9000 = 250C2
C2 = 36% m/v
Therefore, the concentration of the resulting antibiotics solution that must be prepared by diluting 100mL of a 90. % m/v solution and bringing up the volume to 250 mL is 36% m/v.
Learn more about concentration at: https://brainly.com/question/202460
#SPJ6
U Activity 2. Lights On, Lights Off Write On if the process pertains to light-dependent reaction and writes OFF if the process pertains to the light-independent reaction. Write your answer on a separate sheet of paper.
1. It is also known as the dark reaction of photosynthesis.
2. Primary acceptor of carbon is Photosystem I and II.
3. Site of the process is in the stroma. 4. Photolysis of water does not occur.
5. Process type is both cyclic and non-cyclic processes.
6. It is a release of oxygen that gives off aldehydes and hydrogen upon dehydrogenation
7. It is a process that converts solar energy into chemical energy.
8. It is a light dependent process.
9. Process type is cyclic only,
10. Primary acceptor of carbon is Rubiscobisphosphate.
Answers
1. It is also known as the dark reaction of photosynthesis: Off.
A dark reaction of photosynthesis takes place outside of the thylakoids and do not require light to proceed, so it is a light-independent reaction.2. Primary acceptor of carbon is Photosystem I and II: On.
Photosystem I (P700) and Photosystem II (P680) are large membrane protein complexes that accepts carbon during a light-dependent reaction.3. Site of the process is in the stroma: Off.
The stroma is the site for series of biochemical redox reactions called Calvin cycle, which is a light-independent reaction.4. Photolysis of water does not occur: Off.
Since the photolysis of water doesn't occur, the reaction is a light-independent reaction.5. Process type is both cyclic and non-cyclic processes: On.
A non-cyclic processes forms ATP, so it is a light-dependent reaction.6. It is a release of oxygen that gives off aldehydes and hydrogen upon dehydrogenation: On.
A light-dependent reaction causes a release of oxygen that gives off aldehydes.7. It is a process that converts solar energy into chemical energy: On.
The conversion of solar energy into chemical energy is typically a light-dependent reaction.8. It is a light dependent process: On.
A light-dependent reaction takes place in the presence of light.9. Process type is cyclic only: Off.
A cyclic process is a light-independent reaction because it doesn't require light.10. Primary acceptor of carbon is Rubiscobisphosphate: Off.
When Rubiscobisphosphate is the primary acceptor of carbon, the reaction is a light-independent reaction.Read more: https://brainly.com/question/12827458
How does pollution affect Earth Systems.
ILL GIVE BRAINLIEST!!!!!!
Answers
Answer:
pollution causes smog
Smoke fog, or smog for short, is a type of intense air pollution.
it makes GHG wich is greenhouse gas and it makes the smog to stay inside the earth wich heats the Earth and it causes flood, storm, tornadoes, ect.
which climate is it marking brainliest

Answers
Answer:
D highland
Explanation:
Answer:
A or C
Explanation:
it's one of those answers I'm sure of
Lab: Thermal Energy Student Guide Table C PLEASE HELP!!!!
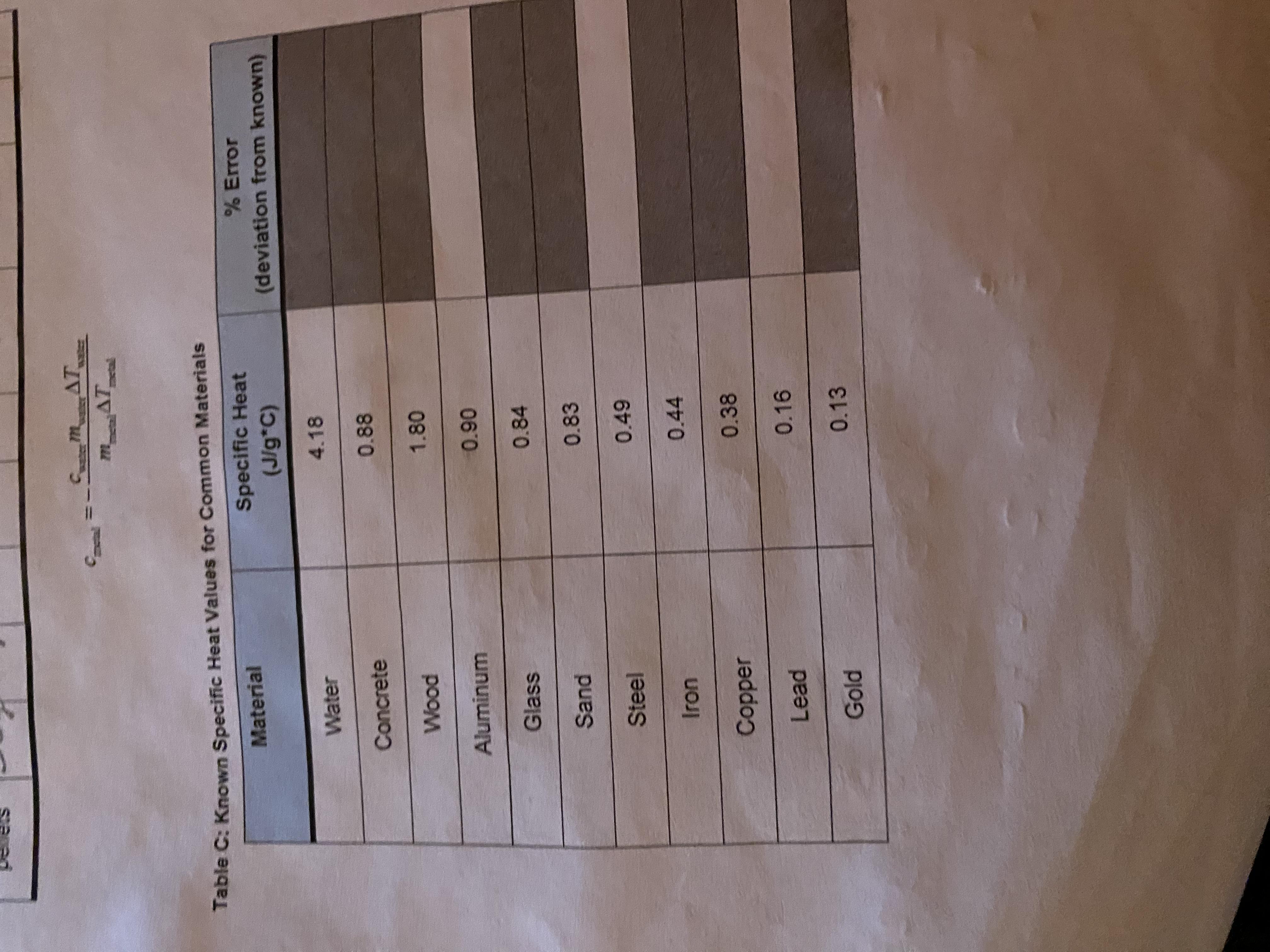
Answers
Based on the data, the missing error percentages would be 0%, 13.6%, -2.22%, and -10.7%.
What is an error percentage?The error percentage as indicated by its name shows in a percentage, the difference between an estimated value and the real value or value measured during an experiment. Based on this, in this case, you are required to find the difference between the value registered in the table for each material and the standard. The general formula for this is:
Percentage Error = ((Standard – Actual Number)/ Standard) x 100.
Water: 4.18 vs 4.18 = 0%
Concrete: 0.88 vs 1 = 13.6%
Wood: 1.80 vs 1.76 = -2.22%
Glass: 0.84 vs 0.75 = -10.7%
Sand: 0.83 vs 0.83 = 0%
Steel: 0.49 vs 0.42 = -14.2%
Iron: 0.44 vs 0.45 = 2.27%
Cooper: 0.38 vs 0.38 = 0%
Lead: 0.16 vs 0.12 =-25%
Gold: 0.13 vs 0.03 = -76.9%
Learn more about error in brainly.com/question/19575648
#SPJ1
What is the numerical value for the standard cell potential of the following reaction?
2Cr2(aq) + 3Cu(s) → 2Cro) + 3Cu2+ (aq)
a) -1.25 V
b) -0.57 V
c) +0.57 V
d) +1.25 V
Answers
Hope this help!
Prepare one solution that has 0.12 M of FeCl3 and 0.40 M of HCl with the reagents 3 M HCl and Solid FeCL3 * 6H20. Provide the calculations and protocol to make the solution in a lab.
Answers
To prepare a 0.12 M solution of FeCl₃, the amount of solid FeCl₃ to be dissolved in a given volume of solvent will be 9.72 grams.
Given,
Molarity of FeCl₃ (M)= 0.12 M
The molecular weight (m) of FeCl₃ is = 162 gm
The volume of the solution (V) to be prepared is =500 ml
The amount of FeCl₃ to be dissolved to make a 0.12 M solution is= x
So,
MV= x ÷ m × 1000
0.12× 500 = x ÷ 162 × 1000
x = 60 × 162 ÷ 1000
x= 9.72 gm
So 9.72 grams of FeCl₃ is dissolved to make 500 ml of 0.12 M solution.
For preparing 0.4 M HCl from 4M HCL:
If we need to make 500 ml of solution with 0.4M of HCL, then we use the formula:
M₁V₁= M₂V₂
0.4 × 500= 4 × x
x= 50 ml
So 50 ml of 4M HCL is taken to make 0.4 M HCL.
To learn more about FeCl₃, refer to the link:
https://brainly.com/question/32098087
#SPJ1
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
how many moles are in 22 grams of argon
Answers
Answer:
0.551 moles
Explanation:
To calculate the number of moles in 22 grams of argon, divide the mass by the molar mass:
Number of moles = Mass / Molar mass
Number of moles = 22 g / 39.95 g/mol
Number of moles ≈ 0.551 moles
Therefore, there are approximately 0.551 moles of argon in 22 grams of argon.
Show the Schematic representation of a polarimeter?
Answers
A polarimeter fundamental working principle entails the following: By passing light via a polarizer, one can produce light with a precisely prepared linear polarisation state.
What are the uses of polarimeter?Polarimeters have a wide range of uses, including determining the concentration and purity of chemicals in medications, maturation testing for agricultural products, and measuring the sugar content in beverages and candies.A polarimeter is made up of a light source, a monochromator (which reduces the light's spectrum to just one wavelength), a polarizer (which turns the beam of light into plane polarised light), a sample tube (which holds the sample being measured), a second polarizer (which determines the degree of rotation), and a light detector.By directing monochromatic light through two polarising plates, polarimeters calculate the optical activity. Sodium light is utilised because it has a high energy output and provides monochromatic light with a wavelength of 589 nm.To learn more about polarimeter refer to:
https://brainly.com/question/14687806
#SPJ1
What type of energy is transferred during heating?
A. Thermal
B. Temperate
C. Convective
D. Conductive
Thank u for helping !:)
Answers
Answer:
A.) Thermal
Explanation:
I got it correct on founders edtell
22. What is the mass in grams of each of the following?
a. 3.011 x 1023 atoms F
b. 1.50 x 1023 atoms Mg
c. 4.50 x 1012 atoms Cl
d. 8.42 x 1018 atoms Br
e. 25 atoms W
f. 1 atom Au
Answers
The mass in grams of 3.011 x 10²³ atoms of F is 9.5 g.
The mass in grams of 1.50 x 10²³ atoms of Mg is 5.98 g.
The mass in grams of 4.50 x 10¹² atoms of Cl is 2.65 x 10⁻¹⁰ g.
The mass in grams of 8.42 x 10¹⁸ atoms of Br is 1.12 x 10⁻³ g.
The mass in grams of 25 atoms of W is 3.1 x 10⁻²¹ g.
The mass in grams of 1 atom of Au is 3.27 x 10⁻²² g.
What is the mass in grams of 3.011 x 10²³ atoms F?
The mass in grams of 3.011 x 10²³ atoms of F is calculated as follows;
6.023 x 10²³ atoms = 19 g of F
3.011 x 10²³ atoms F = ?
= (3.011 x 10²³ x 19 g)/(6.023 x 10²³)
= 9.5 g
The mass in grams of 1.50 x 10²³ atoms of Mg is calculated as follows;
6.023 x 10²³ atoms = 24g of Mg
1.5 x 10²³ atoms F = ?
= (1.5 x 10²³ x 24 g)/(6.023 x 10²³)
= 5.98 g
The mass in grams of 4.50 x 10¹² atoms of Cl is calculated as follows;
6.023 x 10²³ atoms = 35.5 g of Cl
4.5 x 10²³ atoms Cl = ?
= (4.5 x 10¹² x 35.5 g)/(6.023 x 10²³)
= 2.65 x 10⁻¹⁰ g
The mass in grams of 8.42 x 10¹⁸ atoms of Br is calculated as follows;
6.023 x 10²³ atoms = 80 g of Br
8.42 x 10¹⁸ atoms Br = ?
= (8.42 x 10¹⁸ x 80 g)/(6.023 x 10²³)
= 1.12 x 10⁻³ g
The mass in grams of 25 atoms of W is calculated as follows;
6.023 x 10²³ atoms = 74 g of W
25 atoms W = ?
= (25 x 74 g)/(6.023 x 10²³)
= 3.1 x 10⁻²¹ g
The mass in grams of 1 atom of Au is calculated as follows;
6.023 x 10²³ atoms = 197 g of Au
1 atom of Au = ?
= (1 x 197 g)/(6.023 x 10²³)
= 3.27 x 10⁻²² g
Learn more about atomic mass here: https://brainly.com/question/338808
#SPJ1
Use the graph to answer these questions. For
numerical answers, use correct significant digits.
Letter shows the overall enthalpy of reaction
which is equal to
Letter shows the activation energy for the
reaction.
kJ.
The activated complex is represented by
DONE
6CO₂(g) + 6H₂O(g) → CH₁2O(s) +60₂2(g)
CH₁2O(s) + O₂(g)
-1273.02 kJ
Energy
-3811.92 kJ
6CO₂(g) + 6H₂O(g)
A
Reaction Progression
Answers
Letter A indicates the overall enthalpy of reaction
Which exists equivalent to 2,538.90 kJ
Letter B indicates the activation energy for the reaction
The activated complex exists denoted by letter C
What is meant by the enthalpy of reaction?The change in the enthalpy of a chemical reaction that takes place at constant pressure is known as the Heat of Reaction (also known as the Enthalpy of Reaction). It is a thermodynamic unit of measurement that can be used to determine how much energy is released or created per mole during a reaction.
1. Letter __ shows the overall enthalpy of reaction
The change in enthalpy of the components is equivalent to the total enthalpy of the reaction:
Enthalpy of reaction = ∑ enthalpy of the products - ∑ enthalpy of the reactants.
The products exists shown of the right side of the diagram. They exists C₆H₁₂O₆(g) and 6O₂(g).
The reactants exists shown of the left side of the diagram. They exists 6CO₂(g) and 6H₂O(g).
The arrow labeled A shows the difference between the enthalpies of the products and the reactants; therefore, this shows the overall enthalpy of reaction.
2. which is equal to kJ__
The initial state's reactants' enthalpy must be subtracted from the final state's enthalpy (products).
Enthalpy of reaction as a whole: 1,273.02kJ ( - 3,811.92kJ)
Enthalpy of the entire reaction is 2,538.90 kJ.
This indicates that the reaction is endothermic because of the positive change.
3. Letter ___ shows the activation energy for the reaction
The energy that the reactants must acquire for the reaction to take place is known as the activation energy.
Therefore, it is the difference between the starting state and the maximum energy, which is denoted by the label C on the graph.
The arrow labeled B illustrates this: for the reactants to achieve the energy of the state labeled C, they must gain the energy indicated by the arrow labeled B.
4. The activated complex is represented by ____
The substance known as the activated complex is created as the reactants break their connections and begin to make new bonds in the products. The term "transition state" is sometimes used.
The activated complex has the maximum energy, hence it is at the top of the energy diagram. Energy is always needed for the activated complex to develop (it is an endothermic process). Energy is released after it forms, and the reactants are created (this part is exothermic).
Therefore, the activated complex exists labeled with the letter C.
The complete question is:
Use the graph to answer these questions. For numerical answers, use correct significant digits.
Letter __ shows the overall enthalpy of reaction
which is equal to kJ__
Letter ___ shows the activation energy for the reaction
The activated complex is represented by
To learn more about activated complex refer to:
https://brainly.com/question/4358233
#SPJ9

Oxygen and chlorine gas are mixed in a container with partial pressures of 3040 mmHg and
1.63 atm, respectively.
What is the TOTAL pressure inside the container in atm? *Don't forget to convert: 760mmHg
= 1atm*
Answers
Answer:5.63 atm
Explanation: Dalton's Law states that total pressure of a mixture of gasses is equal to the sum of partial pressure of each gas.
\(P_{tot} = P_{1} +P_{2}+ P_{3}...\)
We are given pressure O2 and pressure Cl2.
Step 1: First, convert 3040mmHg to atm by dividing by 760.
\(\frac{3040mmHg}{760atm} = 4 atm\)
Step 2: Plug your numbers into the equation
\(P_{tot} = P_{O2} +P_{Cl2}\)
\(P_{tot} = 4atm + 1.63 atm = 5.63 atm\)
Waves conduct energy through
Answers
Calculate the relative molecular mass of hydrated Copper (II) tetraoxosulphate (VI) CuSO4.5H₂O (Cu = 64 S = 32 H = 1 0 = 16).
Answers
The relative molecular mass of hydrated Copper (II) tetraoxosulphate (VI) CuSO4.5H₂O is 249.
What is molecular mass?Molecular mass is a measure of the total mass of one mole of a substance, which is defined as the mass of the substance divided by the number of molecules it contains. It is typically expressed in g/mol and is also known as molar mass. Molecular mass is determined by the types and number of atoms that compose a molecule, and is an important factor in understanding the properties of a substance.
This is calculated by adding the atomic masses of all the atoms present in the compound.
The atomic mass of copper is 64, sulphur is 32, oxygen is 16, and hydrogen is 1.
So, the relative molecular mass of hydrated Copper (II) tetraoxosulphate (VI) CuSO4.5H₂O is 64 + 32 + (16*4.5) + (1*5) = 249.
To learn more about molecular mass
https://brainly.com/question/24727597
#SPJ1
Chemistyd
By mistake you and sat instead of sugar to the
of How can you remove the salt
Answers
If you accidentally add salt instead of sugar to a recipe, you can use vinegar to counteract the salty taste.
If you have added salt instead of sugar to a recipe, then you can try to remove the salt by adding a substance that will counteract its flavor. One such substance is vinegar, which is an acid and can help to neutralize the salty taste. Here are the steps to remove salt from a dish:
1. Remove as much of the salty liquid or sauce as possible.
2. Dilute the remaining sauce or liquid by adding more of the ingredients in the recipe, except for the salt
3. Taste the dish and add more sugar if needed.
4. If the dish is still too salty, add a little bit of vinegar.
5. Keep tasting the dish and adjusting the sugar and vinegar until it is no longer too salty.6. If the dish becomes too sweet, add more of the other ingredients to balance it out.
Know more about salt here:
https://brainly.com/question/20835655
#SPJ8
What is the maximum mass of tungsten that can be formed with 200g of tungsten oxide?
WO3 + 3H2 —-> W + 3H2O
Answers
WO3 + 3H2 → W + 3H2O
From the equation, we can see that 1 mole of WO3 reacts to form 1 mole of W. To calculate the maximum mass of tungsten, we need to convert the given mass of WO3 to moles, and then use the mole ratio to determine the mass of W.
First, we need to determine the molar mass of WO3. Tungsten (W) has a molar mass of 183.84 g/mol, and oxygen (O) has a molar mass of 16.00 g/mol. Since WO3 has one tungsten atom and three oxygen atoms, the molar mass of WO3 is:
Molar mass of WO3 = (1 * molar mass of W) + (3 * molar mass of O)
= (1 * 183.84 g/mol) + (3 * 16.00 g/mol)
= 183.84 g/mol + 48.00 g/mol
= 231.84 g/mol
Next, we can calculate the number of moles of WO3 using the given mass:
Number of moles of WO3 = Mass of WO3 / Molar mass of WO3
= 200 g / 231.84 g/mol
≈ 0.862 mol
Since the mole ratio between WO3 and W is 1:1, the number of moles of tungsten (W) formed will also be 0.862 mol.
Finally, we can calculate the mass of tungsten (W) using the molar mass of tungsten (183.84 g/mol):
Mass of tungsten (W) = Number of moles of W * Molar mass of W
= 0.862 mol * 183.84 g/mol
≈ 158.56 g
Therefore, the maximum mass of tungsten that can be formed from 200g of tungsten oxide (WO3) is approximately 158.56 grams.
Question:
Define the following terms associated with titration:
A) Standard solution
B) Endpoint
C) Indicator
Titration
Chemical titrations are reactions performed to determine some property of a solution. In a titration experiment, there are two solutions. In one solution a property is known, and that property is used to discover something about the other solution. Often, a preset volume of the known solution is required to bring about a certain change in the unknown solution.
Answers
A titration is a method where the concentration of an unknown solution is ascertained by comparing it to a solution of known concentration.
What is the term titration?Titration is a method of chemical analysis where the amount of a sample's ingredient is determined by adding an exact known amount of a different substance to the measured sample, which the desired constituent reacts with in a specific, known proportion.
An acid-base titration involves gradually adding an unknown base to an acid solution with a known concentration (a standard solution) (or vice versa). To the base, you add a few drops of indicator solution. When the base has been neutralized (when [H+] Equals [OH-]), the indicator's color will change to indicate this. A substance's color changing due to a chemical reaction is an indicator. The pH affects the color of an acid-base indicator, such as phenolphthalein. It is also used redox indicators.
To learn more about titration refer to :
https://brainly.com/question/186765
#SPJ4
Identify the possible issues if a sample in a spectrophotometer gives no reading. Select one or more: The wrong wavelength may be set. There may be an issue with the spectrophotometer. The sample may be placed impro
Answers
The question is incomplete; the complete question is;
Identify the possible issues if a sample in a spectrophotometer gives no reading. Select one or more:
The wrong wavelength may be set.
The sample may be placed improperly in the cuvette holder.
There may be an issue with the composition of the sample.
There may be an issue with the spectrophotometer
Answer:
The wrong wavelength may be set.
There may be an issue with the spectrophotometer
Explanation:
Substances do not absorb radiation at all wavelengths. The proper wavelength at which a substance absorbs must be used for a reading to be obtained from the spectrophotometer. If this is not done, no reading is obtained from the spectrophotometer.
Generally, if the spectrophotometer has an issue, it may display no reading until the machine is fixed.
The possible issues if a sample in a spectrophotometer gives no reading are:
The sample may be placed improperly in the cuvette holder. The wrong wavelength may be set.According to the given question, we are asked to identify the possible reasons why a spectrophotometer would give no reading when a sample is used on it.
As a result of this, it is important to note that it is possible that the sample may not have been placed correctly on the cuvette holder or the wrong wavelength may be set which would cause the no reading error to show.
Read more here:
https://brainly.com/question/13998813
What is one property for each of the chemical substances listed above?
1. Acetic acid-
2. Sodium bicarbonate-
3. Carbon dioxide-
4.Dihydrogen monoxide-
5. Sodium acetate-
6. Calcium chloride-
7. Calcium carbonate-
8. Sodium chloride-
Answers
2. Sodium Bicarbonate is an odorless, crystalline powder.
3. Carbon dioxide is a colorless gas.
4. Dihydrogen monoxide is colorless and odorless gas.
5. Sodium acetate is very soluble in water.
6. Calcium chloride has an exothermic reaction when dissolved in water.
7. Calcium carbonate is also an odorless powder.
8. Sodium chloride is soluble in water, and partially soluble (or insoluble) in other liquids.
Hope this helps! (:
what is the change in mass of A in
60 minutes?
Mass of A (g)
12.4
10.4
9.1
7.7
6.2
Time
O
15
30
45
60
Answers
Answer:
To determine the change in mass of A over the given time period, we need to find the difference between the initial mass of A and the final mass of A.
From the given table, we can see that the initial mass of A at t = 0 (start time) is 12.4 g and the final mass of A at t = 60 minutes (end time) is 6.2 g.
Therefore, the change in mass of A over 60 minutes is:
Final mass of A - Initial mass of A
= 6.2 g - 12.4 g
= -6.2 g
The negative sign indicates that the mass of A decreased over time, which means that A underwent some kind of reaction or process that caused it to lose mass.
The change in mass of A over 60 minutes is -6.2 grams.
To determine the change in mass of A over 60 minutes, we need to compare the initial mass to the final mass.
From the given information, we can see that the mass of A decreases over time.
Let's calculate the change in mass.
Initial Mass of A: 12.4 g
Final Mass of A: 6.2 g
Change in Mass of A = Final Mass of A - Initial Mass of A
= 6.2 g - 12.4 g
= -6.2 g
The change in mass of A over 60 minutes is -6.2 grams.
Note that the negative sign indicates a decrease in mass.
For such more questions on mass
https://brainly.com/question/1838164
#SPJ8
Write the empirical formula of at least four binary ionic compounds that could be formed from the following ions:
Ca^2+, Cr^4+, Cl^-, S^2-
Answers
Answer:
\($ CaCl_2$\), CaS, \($ Cr Cl_4$\), \($ CrS_2$\)
Explanation:
The reaction can be done by adding the cations and the anions.
The binary compounds are always formed by the two different elements.
When one calcium ion, \($ Ca ^{2+} $\) combines with the two calcium ions, \($ Cl^-$\), they formed a binary compound of \($ CaCl_2$\).
When one \($ Ca ^{2+} $\) combines with one \($S^{2-}$\) ion, they formed CaS binary compound.
When one chromium ion \($ Cr^{4+}$\) combines with four ions of chlorine, \($ Cl ^- $\), the compound formed is \($ Cr Cl_4$\).
When 1 ion of \($ Cr^{4+}$\) combines with 2 ions of sulphur, \($S^2$\), the binary compound formed is \($ CrS_2$\).
What type of compound is represented by the graph at right? A. strong base B. strong acid C. weak base D. weak acid

Answers
The type of compound represented by the graph at right is a strong acid (option B).
What is a strong acid?An acid is generally any compound capable of dissociating into its respective constituent ions when in an aqueous solution.
An acid is categorised as strong or weak depending on whether it can dissociate completely or partially. A strong acid dissociates completely in water.
According to this question, HA, when added to water, dissociates into H+ and A- ions, hence, is a strong acid.
Learn more about strong acid at: https://brainly.com/question/29769012
#SPJ1
According to Coulomb's law, what will happen to the electric force between two identical negative charges as they move closer together?
Answers
Answer:
According to Coulomb’s law, the electric force between two identical negative charges is inversely proportional to the square of the distance between them. This means that as the distance between the two charges decreases, the electric force between them will increase. Since the charges are both negative, they will repel each other, so as they move closer together, the repulsive force between them will become stronger.
Explanation: