Which of these is NOT correct?1. Kinases are enzymes that phosphorylate other molecules.2. Cyclic AMP binds to calmodulin.3. Ion channels are found on both the plasma membrane and the endoplasmic reticulum.4. Tyrosine-kinase receptors consist of two polypeptides that join when activated by a signal molecule.5. Phospholipase C catalyzes the formation of IP3.
Answers
False: Cyclic AMP binds to calmodulin Calcium binds to calmodulin is the appropriate response.
This protein-protein interaction ultimately has a physiological consequence; for instance, in smooth muscle, Ca2+ binding to calmodulin causes it to connect with and activate myosin light chain kinase, which in turn catalyses the phosphorylation of myosin. The smooth muscle contracts as a result of this reaction.
The protein calmodulin interacts with calcium ions as they enter a cell. Four calcium sites are present in this protein, and when three or four of these sites are occupied by calcium, the calmodulin changes form and starts a number of internal cell processes, such as the activation or inhibition of protein kinases.
To learn more about Calcium binds to calmodulin. Please visit the below link.
https://brainly.com/question/14351855
#SPJ4
Related Questions
Aqueous copper (II) nitrate, Cu(NO3)2 reacts with aqueous potassium carbonate, K2CO3 forming solid copper (II) carbonate, Cu(CO3).
Answers
Answer:
Cu(NO3)2 (aq) + K2CO3 (aq) => CuCO3 (s) + 2KNO3 (aq)
Explanation:
I interpret your question is how to balance the above reaction, right?
Cu(NO3)2 (aq) + K2CO3 (aq) => CuCO3 (s) + 2KNO3 (aq)
Cu: 2
NO3: 2
K: 2
CO3: 2
The boiling point of another member of this homologous series was found to be 309 KK. What is the likely molecular formula for this compound?
Answers
Answer: Pentane C5H12
Explanation:
The boiling point of a substance is simply defined as the temperature whereby a liquid's vapor pressure is equal to the pressure that is surrounding the liquid and hence, the liquid will changes into vapor.
The likely molecular formula for this compound is Pentane i.e C5H12 due to the fact that its boiling point is between Butane with formula C4H10 and Hexane with formula C6H14 boiling points.
The molecular formula of the of the substance is \(\bold {C_5H_1_0}\) (pentane).
Boiling point:
It is the temperature at which liquid's vapor pressure is equal to the pressure that is surrounding the liquid and hence, the liquid start to into vaporize.
The boiling point of the given compound is 309 K which is between Butane and Hexane.
Therefore, the molecular formula of the of the substance is \(\bold {C_5H_1_0}\) (pentane).
To know more about Boiling point,
https://brainly.com/question/2153588
A gas sample weighing 3.78 grams occupies a volume of 2280 mL at STP. What is the apparent molecular mass of the sample?
(Note: R = 8.314 kPa.dm3/K.mol or 0.0821 L.atm/K.mol or 62.4 L.torr/K.mol)
Answers
PV = nRT
where:
P is the pressure of the gas (at STP, P = 1 atm)
V is the volume of the gas (2280 mL = 2.280 L)
n is the number of moles of gas
R is the ideal gas constant (0.0821 L.atm/K.mol)
T is the temperature of the gas (at STP, T = 273.15 K)
We can rearrange the ideal gas law to solve for n:
n = PV/RT
Let's plug in the given values and solve for n:
n = (1 atm) * (2.280 L) / (0.0821 L.atm/K.mol * 273.15 K)
n = 0.1002 mol
Now we can calculate the apparent molecular mass of the gas sample using the formula:
molecular mass = mass / moles
Let's plug in the given values and solve for the molecular mass:
molecular mass = 3.78 g / 0.1002 mol
molecular mass = 37.7 g/mol
Therefore, the apparent molecular mass of the gas sample is approximately 37.7 g/mol.
A cylinder is filled to a volume of 3.50 L at 760 mmHg with chlorine gas. What is the volume if the pressure is changed to 50.0 kPa?
Answers
When a cylinder is filled to a volume of 3.50 L at 760 mmHg with chlorine gas. The volume of the gas if the pressure is changed to 50.0 kPa is 7.0924 L.
Boyle's law is generally a gas law which states that "a gas's pressure and volume are inversely proportional when the temperature is kept constant, as volume increases, pressure falls and vice versa".
Mathematically,
P₁V₁ = P₂V₂
P₁ = 760 mmHg = 101.32 kPa
V₁ = 3.50 L
P₂ = 50 kPa
V₂ = ?
Substituting the values we get,
101.32 × 3.50 = 50 × V₂
⇒ V₂ = (101.32 × 3.50)/50
⇒ V₂ = 7.0924 L
Hence, the volume of the gas if the pressure is changed to 50.0 kPa is 7.0924 L.
Learn more about Boyle's law from the link given below.
https://brainly.com/question/30367133
#SPJ1
The half-life of a radioactive isotope is 27 years. How long will its
mass take to fall from 2.00 g to 0.25 g?
years.
Answers
Life will come out to be 10 days. This is the required value of half life.
what is radioactive isotope?
An unstable form of a chemical element that releases radiation as it breaks down and becomes more stable. Radioisotopes may occur in nature or be made in a laboratory. In medicine, they are used in imaging tests and in treatment. Also called radionuclide. Radioactive isotopes have many useful applications. In medicine, for example, cobalt-60 is extensively employed as a radiation source to arrest the development of cancer. Other radioactive isotopes are used as tracers for diagnostic purposes as well as in research on metabolic processes.Atoms of the same element with different numbers of neutrons are called isotopes. Many elements have one or more isotopes that are radioactive. Their nuclei are unstable, so they break down, or decay, and emit radiation.To learn more about isotope refers to:
https://brainly.com/question/364529
#SPJ1
help please hurry lots of points
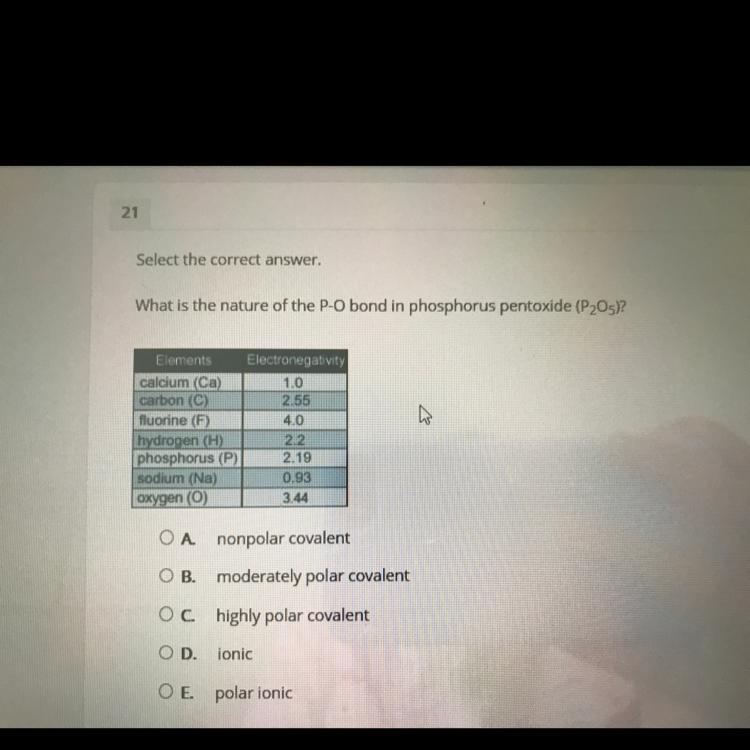
Answers
which type of soil is the best for planting? A.Loam B.Clay c.sand
Answers
Answer:
A. Loam soil is best for planting
Thank you....
Have a good day....
The equilibrium constant, Kc, for the following reaction is 4.76×10-4 at 431 K. PCl5(g) PCl3(g) + Cl2(g) When a sufficiently large sample of PCl5(g) is introduced into an evacuated vessel at 431 K, the equilibrium concentration of Cl2(g) is found to be 0.233 M. Calculate the concentration of PCl5 in the equilibrium mixture. M
Answers
Answer:
Explanation:
Step 1: Data given
The equilibrium constant, Kc, for the following reaction is 4.76 * 10^-4 at 431 K
The equilibrium concentration of Cl2(g) is 0.233 M
Step 2: The balanced equation
PCl5(g) ⇄ PCl3(g) + Cl2(g)
Step 3: The initial concentration
[PCl5]= Y M
[PCl] = 0M
[Cl2] = 0M
Step 4: Calculate the concentration at equilibrium
[PCl5] = Y + X M = Y - 0.233 M
[PCl]= XM = 0.233 M
[Cl2]= XM = 0.233 M
Step 5: Define Kc
Kc = [Cl2]* [PCl3] / [PCl5]
4.76 * 10^-4 = 0.233² / (Y -0.233)
0.000476 = 0.05429 / (Y - 0.233)
Y - 0.233 = 0.05429 / 0.000476
Y - 0.233 = 114.05 M
Y = 114.283 M = the initial concentration
The concentration of PCl5 at the equilibrium is 114.05 M
h. Identity of unknown metal
Answers
Answer:
you can identify an unknown substance by measuring its density and comparing your results to a list of known densities. Density=mass/volume. Assume that you have to identify an unknown metal. You can determine the mass of the metal on a scale.
If 42.6 grams of Al reacts completely with O2 and 57.8 grams of Al2O3 produced, what is the % yield of Al2O3for the reaction? 4Al + 3O2 --------> 2Al2O3Group of answer choices22.3%65.9%55.7%75.2%71.8%
Answers
Step 1
Data provided:
The reaction: 4Al + 3O2 => 2Al2O3 (balanced)
The limiting reactant Al (it is said that Al reacts completely)
Mass of Al = 42.6 g
The actual yield = 57.8 g Al2O3
-----------------------
Step 2
Data needed: the molar mass of Al (26.9 g/mol) and Al2O3 (101.9 g/mol)
The theoretical yield:
4 x 26.9 g Al ------- 2 x 101.9 g Al2O3
42.6 g Al ------- X = 80.7 g Al2O3 = theoretical yield
---------------------
Step 3
% yield = (actual yield/theoretical yield) x 100 = (57.8 g/80.7 g) x 100 = 71.6 %
(this is the closest value for one of the options, 71.8 %)
Answer: 71.8 %
Which of the following fractions can be used for the mole ratio to determine the mass of Cl2 from a known mass of AlCl3?
1) 2/3
2) 1/2
Answers
The correct fraction that can be used for the mole ratio to determine the mass of Cl2 from a known mass of AlCl3 is 2/2.
What is the mole ratio?The mole ratio is a way of expressing the ratio of the number of moles of one substance in a chemical reaction to the number of moles of another substance in the same reaction.
Mole ratio is determined by the stoichiometry of the reaction, which is the quantitative relationship between the amounts of reactants and products in a chemical equation. The coefficients in a balanced chemical equation represent the mole ratios of the reactants and products in the reaction.
Learn more about mole ratio:https://brainly.com/question/15288923
#SPJ1
Missing parts;
Read the following chemical equation.
Al + Cl2 → AlCl3
Which of the following fractions can be used for the mole ratio to determine the mass of Al from a known mass of AlCl3?
A. 2/1
B. 3/2
C. 2/2
D. 2/3
Do lone pair electrons form covalent bonds YES or No
Answers
Answer:
yeslone pair of electrons form covalent bonds
Explanation:
Hope it helps you<3IPA is extracted from the IPA-cyclohexane mixture containing 40% IPA in a countercurrent extraction unit using water. The amount of water in the feed
its mass ratio to the amount of oil is 5.25 and the balance data are given in the figure below. The ideal number of racks required for the final raffin to contain 20% IPA and the % of the first extract.
Determine its composition.
Answers
To answer your question, we will need to utilize the given information and perform calculations using the provided terms, such as the IPA-cyclohexane mixture, countercurrent extraction unit, mass ratio, and ideal number of racks.
First, let's find the initial composition of the mixture:
- 40% IPA (isopropanol)
- 60% Cyclohexane
Now, using the given mass ratio of water to oil (5.25), we can calculate the amounts of water and oil in the feed. Since we don't have exact values for the amounts, let's assume there are 100 units of the mixture.
- Water: (5.25 * 100) / (5.25 + 1) ≈ 84.0 units
- Oil: 100 units
The countercurrent extraction unit uses water to extract the IPA from the mixture. The objective is to achieve a final raffinate containing 20% IPA.
To determine the ideal number of racks and the composition of the first extract, we would need the provided balance data figure, which is not available in the question. However, by following the steps below, you can determine the values using the balance data figure:
1. Locate the initial point on the balance data figure, corresponding to the 40% IPA composition in the mixture.
2. Draw a tie line connecting the initial point to the mass ratio line (5.25) on the figure.
3. Identify the intersection point of the tie line with the mass ratio line, which represents the composition of the first extract.
4. Calculate the number of ideal racks by drawing a series of tie lines and steps from the initial point towards the final raffinate point (20% IPA) on the balance data figure.
By following these steps and using the provided balance data figure, you can determine the ideal number of racks required for the countercurrent extraction unit and the composition of the first extract.
For more questions on: extraction
https://brainly.com/question/28976060
#SPJ11
what causes deep ocean currents to flow
Answers
Answer:Deep ocean currents (also known as Thermohaline Circulation) are caused by: ... The sinking and transport of large masses of cool water gives rise to the thermohaline circulation, which is driven by density gradients due to variations in temperature and salinity. The earth's rotation also influences deep ocean currents.
Explanation:
Write a balanced equation for the decomposition reaction that occurred in Experiment 2. Include physical states.
Answers
Classify each substance as an atomic element, molecular element, molecular compound, or ionic compound.
Drag the appropriate items to their respective bins.
oxygen, iron, CaO, N2H4, Fe2O3
Answers
Answer:
element ; oxygen and iron
ionic compound ; cao ,fe2o3
molecular compound; N2H4
what is photosynthesis
Answers
Answer:
the process by which green plants and some other organisms use sunlight to synthesize foods from carbon dioxide and water. Photosynthesis in plants generally involves the green pigment chlorophyll and generates oxygen as a byproduct.
Explanation: You can put this in your own words. How? Well, try to find the most important stuff in your own words and write it down.
HELP!!!
Which of the following is an example of a chemical change?
А
cutting paper
B
ice melting in a glass
c
wood burning in a fireplace
D
defrosting food in the microwave

Answers
Answer:
C. Burning wood
Explanation:
BURNING. Burning is a non-reversible chemical change. When you burn wood, the carbon in the wood reacts with oxygen in the air to create ash and smoke, and energy in the form of light and heat. This is a permanent change that cannot be undone – you cannot turn ashes back into wood.
how many moles of Cl2(g) will be present at equilibrium?

Answers
The moles of Cl₂(g) will be present at equilibrium is 3.94 × 10⁻⁴ mol
To determine the number of moles of Cl₂(g) at equilibrium, we need to use the given equilibrium constant (Kₑ) and the initial concentrations of CO(g) and COCl₂(g).
Given:
[CO(g)] = 0.3500 mol
[COCl₂(g)] = 0.05500 mol
Kₑ = 1.2 × 10²
The balanced equation for the reaction is:
CO(g) + Cl₂(g) ⇌ COCl₂(g)
Let's denote the number of moles of Cl₂(g) at equilibrium as x. At the start, we have [Cl₂(g)] = 0 mol, but it will change by x at equilibrium.
Using the equilibrium expression, we can write:
Kₑ = [COCl₂(g)] / ([CO(g)] * [Cl₂(g)])
Plugging in the values:
1.2 × 10² = [COCl₂(g)] / (0.3500 * [Cl₂(g)])
To solve for [Cl₂(g)], we need to isolate it on one side of the equation:
[Cl₂(g)] = [COCl₂(g)] / (0.3500 * Kₑ)
Now, let's substitute the given values:
[Cl₂(g)] = 0.05500 / (0.3500 * 1.2 × 10²)
[Cl₂(g)] ≈ 3.94 × 10⁻⁴ mol
Therefore, approximately 3.94 × 10⁻⁴ moles of Cl₂(g) will be present at equilibrium.
Know more about moles here:
https://brainly.com/question/29367909
#SPJ8
The question was Incomplete, Find the full content below:
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl₂(g) in a 3.050-L flask at 668 K, how many moles of Cl₂(g) will be present at equilibrium
CO(g) + Cl₂(g) ⇄ COCl₂(g) Ke 1.2 x 10^{2} at 668 K
stoichiometric calculations, including ratios of atoms within a compound, and ratios of substances in a chemical reaction.
Answers
The stoichiometry calculation involves the ratios of atoms within a compound, and ratios of substances in a chemical reaction.
The stoichiometry calculation involves the relationship between the product and the reactants in the chemical rection. stoichiometry is the measurement of the element. the steps follows the stoichiometry calculation is :
1) balance the chemical reaction.
2) convert the substance units to moles.
3) by using the moles ratio to calculate the yield of the substances in the reaction.
4) convert the moles of the needed elements to the required units.
The stoichiometry calculation is mostly based on the chemical formulas of the compound.
to learn more about stoichiometry here
https://brainly.com/question/4066401
#SPJ4
Please help !!!
The atomic number of sodium is 11. How many neutrons does an isotope with a mass number of 22 have ?
Answers
Explanation: The mass number of an element tells us the number of protons AND neutrons in an atom (the two particles that have a measurable mass). Sodium has a mass number of 23amu. Since sodium has 11 protons, the number of neutrons must be 23 – 11 = 12 neutrons.
Answer:
11
Explanation:
How many nerves are in your body to send messages to brain and back? millions or billions
Answers
Answer:
billions
hope this helps :)
Bc they’re a lot of nerves that send in every part if your body
if there are more products than reactants, does that mean there is an increase in the forward or backward reaction? And if there are more reactants that products, is there an increase in the forward or backward reaction?
Answers
Answer:
If there are more products than reactants, that means the reaction has shifted towards the left, which is the backward direction. If there are more reactants than products, that means the reaction has shifted towards the right, which is the forward direction.
A 20.0 ml sample of glycerol has a mass of 25.2 grams. What is the mass of 63 ml sample of glycerol?
Answers
The mass of 63 ml sample : 79.38 g
Further explanationGiven
20 ml and 25.2 g of glycerol
Required
The mass of 63 ml sample
Solution
Density is the ratio of mass per unit volume
Density formula:
\(\large{\boxed {\bold {\rho~=~ \frac {m} {V}}}}\)
Density of glycerol :
= m : V
= 25.2 g : 20 ml
= 1.26 g/ml
Mass of 63 ml sample :
= density x volume
= 1.26 g/ml x 63 ml
= 79.38 g
(12 points total) a researcher prepares a 1.00 m (molal) solution of acetone (ch3coch3) dissolved in ethanol (c2h5oh). given the densities of acetone and ethanol are 0.788 g/ml and 0.789 g/ml, respectively, calculate the concentration values of acetone below. assume that the final volume equals the sum of the volumes of acetone and ethanol. remember to provide units.
Answers
Acetone has a concentration of 0.74 Mm.
What is acetone?Acetone is an organic chemical that is colorless, combustible, volatile, and has a distinctively strong odor. It is a solvent that is frequently used in a variety of commercial and domestic settings. It also serves as a starting point for the synthesis of other chemicals. Acetone has the chemical formula CH3COCH3 and a molecular weight of 58.08 g/mol.
How do you determine it?We must first determine how many moles of acetone are present in the solution.
Per kilogram of solvent, one molal equals one mole of solute.
Since 1 kilogram is equivalent to 1000 milliliters of ethanol, it is possible to compute the number of moles of acetone as follows:
1000 mL/kg times 1.00 mol/kg is 1.00 mol.
The mass of acetone in the solution can then be determined using the molar mass of acetone (58.08 g/mol):
1 mole * 58.08 g/mol = 58.08 g
The volume of acetone can then be calculated by dividing the mass by the density:
58.08 g/0.788 g/mL equals 73.56 mL.
Finally, the molality of acetone can be determined as follows:
0.0074 mol/L (1.00 mol)/1.00 mol/(73.56 mL + 1000 mL) = 0.74 mM.
To know more about acetone, visit:
https://brainly.com/question/13334667
#SPJ4
Multiple Choice
Which of the following is a good study habit?
A. maintaining a positive attitude
B. waiting until the last minute to start on an assignment
C. listening to the television while studying
D. studying when you are tired
Answers
Answer:
A.- maintaining a positive attitude
Explanation:
The answer is very obvious you need to maintain a positive attitude while studying.
Answer:
A: maintaining a positive attitude
Explanation:
I myself have personal experience with all of these, and B,C,D just don´t seem like reasonable answers. If you wait until the last minute to start an assignment, chances are you won´t get it done. Listening to the television while studing is a pretty bad idea because you might get distracted. Never study when tired because if you´re tired the information might not stick in your head. The only answer left is A.
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
which atom has the largest atomic radis
1.potassium
2.iron
3.arsenic
4.bromine
Answers
Answer:
Potassium
Explanation:

Hope this help you!
Phosphorus-32 has a half life of 14.0 days. A 40.0g sample is being shipped. It takes 27 days to arrive, how much P-32 remains?
Answers
The mass of P-32 remaining after 27 days, if the initial mass is 40g is 10.5 g.
What is half life of a radioactive element?
The half-life of a radioactive isotope is the amount of time it takes for one-half of the radioactive isotope to decay.
The half-life of a specific radioactive isotope is constant; it is unaffected by conditions and is independent of the initial amount of that isotope.
The number of half lives in 27 days;
n = 27 days/14 days
n = 1.929
The mass of P-32 remaining after 27 days, if the initial mass is 40g;
mass remaining = 40 g / (2^1.929)
mass remaining = 40 g / 3.808
mass remaining = 10.5 g
Thus, the mass of P-32 remaining after 27 days, if the initial mass is 40g is 10.5 g.
Learn more about half life here: https://brainly.com/question/2320811
#SPJ1
In an experiment, the molar mass of the compound was determined to
be 228.276 g/mol. What is the molecular formula of the compound? Please help!
Answers
For example, a molecule has a molecular weight of 180.18 g/mol. It is found to contain 40.00% carbon, 6.72% hydrogen and 53.28% oxygen.
Convert the percentages to grams.
40.00 grams of carbon
6.72 grams of hydrogen
53.28 grams of oxygen