Answers
Answer:
a option
Explanation:
i think this..... ...
Answer:
A is correct
Hope its help you
Explanation:
Mark me as brainliest.Related Questions
What do the black lines on the map represent?
Answers
Answer:
they show fracture lines shown in geological maps
Explanation:
hope this helps
Which of the following would lose heat the fastest?
15 grams of water
200 grams of water
1000 grams of water
500 grams of water
Answers
Answer:
15 grams of water
Explanation:
15 grams of water of water would lose heat the faster compared to higher masses of water.
Water generally is a poor conductor heat.
To heat up a unit of water, significant amount of energy must be added to the body of water. With time, the body continues to increase in temperature. A 500g mass of water will take more time to lose heat.Zoe left her water bottle capped and in her bedroom. She came back some time later to realize that the bottle was “sweating” and left a ring of liquid on her nightstand
Explain thoroughly the science behind why Zoe’s water bottle is sweating
Answers
Answer:
Condensation
Explanation:
Zoe is quite keen to have noticed what we call condensation. Air contains many components, one of those being water vapor. Like how sugar is soluble in water, water can be said to be "soluble" in air. Water will evaporate into the air to a certain extent. The higher the temperature of the air, the more water the air can hold. If the air has more water that it can hold (potentially because of a temperature decrease), the extra water will come out of the air. Zoe's water bottle was cold, and because the air around Zoe's bottle had cooled down, the air can not hold as much water as it could when it was warm, so the air deposited the extra water in the form of liquid water onto the bottle, giving the illusion that her bottle was sweating.
why electron affinity of 5 th group of periodic table increases down the group?
Answers
The electron affinity of the 5th group increases down the groups of the periodic table because the electrons are placed in a higher energy level far from the nucleus.
What is electron affinity?Electron affinity is the energy released when an electron is attached to an atom or molecule.
Electron affinity is used as a measure of its ability to form an anion.
The electron affinity of elements decrease down the group and increase from left to right across the periodic table because the electrons are placed in a higher energy level far from the nucleus.
Learn more about electron affinity at: https://brainly.com/question/13646318
#SPJ1
a student has a 1 L solution of 2 M HCL and wants to increase the HCL concentration to 3 M
Answers
The student needs to add approximately 83.3 mL of 12 M HCl solution to the existing 1 L of 2 M HCl solution to increase the concentration to 3 M. It is important to handle concentrated acids with caution and follow proper safety procedures.
To increase the concentration of a 1 L solution of 2 M HCl to 3 M, the student needs to calculate the volume of concentrated HCl needed and add it to the existing solution. Here's how the calculation can be done:
Given:
Initial concentration of HCl solution = 2 M
Final concentration desired = 3 M
Initial volume of HCl solution = 1 L
Step 1: Calculate the moles of HCl in the initial solution.
Moles of HCl = Initial concentration × Initial volume = 2 M × 1 L = 2 moles
Step 2: Calculate the moles of HCl needed for the desired concentration.
Moles of HCl needed = Final concentration × Final volume = 3 M × 1 L = 3 moles
Step 3: Calculate the moles of HCl to be added.
Moles of HCl to be added = Moles needed - Moles present = 3 moles - 2 moles = 1 mole
Step 4: Convert the moles of HCl to the required volume of concentrated HCl.
To calculate the volume, we need to know the concentration of the concentrated HCl solution. Assuming it is 12 M, we can use the following formula:
Volume of concentrated HCl = Moles of HCl to be added / Concentration of concentrated HCl
Volume of concentrated HCl = 1 mole / 12 M = 0.0833 L or 83.3 mL
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
Which is a valid velocity reading for an object? 45 m/s 45 m/s north 0 m/s south 0 m/s
Answers
Answer:
45 m/s north
Explanation:
Edge 2020
Answer: (B) 45 m/s north
Explanation: right on edge 2020 (so basically the same reason as person above)
1. As you go across a period, what is a useful comparison point?
A.
oxides
B.
halides
C.
hydrides
D.
hydroxides
-----
2. Most elements are
A.
allotropes
B.
nonmetals
C.
metalloids
D.
metals
----
3. A metalloid is a(n) __ conductor of heat and electricity than a metal.
A.
better
B.
worse
C.
equal
D.
cannot be determined
-----
4. What causes the formation of allotropes?
A.
pressure
B.
light
C.
temperature
D.
all of the above
----
As you move __and __ elements become more metallic.
A.
left, down
B.
right, down
C.
right, up
D.
left, up
------
5. All of the following metalloids form allotropes except
A.
B
B.
Si
C.
Te
D.
Po
------
6. What is a chemical property of a metal?
A.
malleable
B.
ductile
C.
positive oxidation states
D.
conducts heat and electricity
----
Answers
Explanation:
1. As you go across a period, what is a useful comparison point?
A.
oxides
2. Most elements are
A.
allotropes
3. A metalloid is a(n) __ conductor of heat and electricity than a metal.
A.
better
4. What causes the formation of allotropes?
A.
pressure
As you move __and __ elements become more metallic.
A.
left, down
5. All of the following metalloids form allotropes except
A.
B
6. What is a chemical property of a metal?
A.
malleable
A 54.2 g sample of polystyrene, which has a specific heat capacity of 1.880 J-gc, is put into a calorimeter (see sketch at
right) that contains 100.0 g of water. The temperature of the water starts off at 21.0 °C. When the temperature of the water stops
changing it's 34.3 °C. The pressure remains constant at 1 atm.
Calculate the initial temperature of the polystyrene sample. Be sure your answer is rounded to the correct number of significant
digits.
thermometer.
insulated
container
water
sample.
a calorimeter
Answers
Tthe initial temperature of the polystyrene sample is 39.4°C.
Given: Mass of polystyrene sample = 54.2 gSpecific heat of polystyrene = 1.880 J-g°CWater mass = 100.0 g Initial water temperature = 21.0°CWater final temperature = 34.3°CPressure remains constant at 1 atmFormula used:Heat gained by water = heat lost by polystyreneHence,Heat lost by polystyrene = Heat gained by water=> mcΔT = mcΔTwhere,m = mass of polystyrene or waterc = specific heat capacityΔT = change in temperatureThe temperature change is ΔT = 34.3°C - 21.0°C = 13.3°CNow we can use this temperature change to calculate the initial temperature of the polystyrene.Taking the water's specific heat capacity, c = 4.184 J/g°CHeat gained by water = (100.0 g)(4.184 J/g°C)(13.3°C) = 5574 JHeat lost by polystyrene = 5574 JTaking the polystyrene's specific heat capacity, c = 1.880 J/g° ) = 13.3°C Now let's calculate the mass of polystyrene using the specific heat capacity formula.5574 J = (54.2 g)(1.880 J/g°C)(13.3°C - Ti)Ti = 39.4°C
for more questions on polystyrene
https://brainly.com/question/15913091
#SPJ8
What is the role of grasses in the food web above
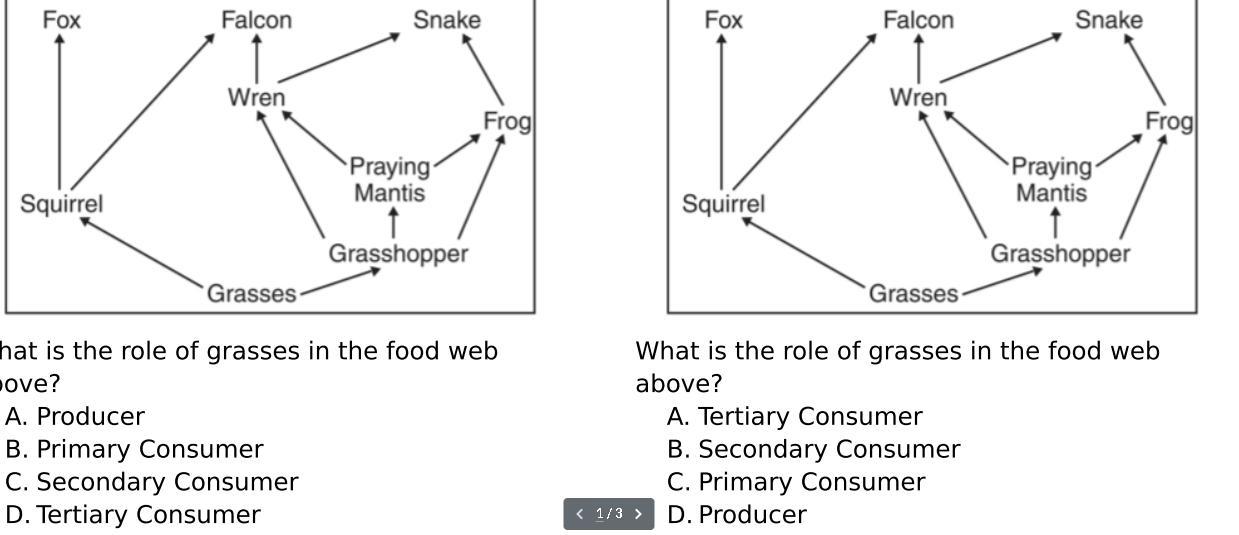
Answers
The food web in the image you provided includes a variety of organisms such as producers, consumers, and decomposers. Grasses play an important role in this food web as they are a primary producer.
What is the process by which grasses and other plants convert sunlight into energy in the food chain?
Grasses, like other plants, use photosynthesis to convert sunlight into energy, which they use to grow and produce organic compounds such as sugars and starches. These compounds form the basis of the food chain, providing energy and nutrients for herbivores such as insects, rodents, and large mammals like deer and bison.
In turn, these herbivores are consumed by predators such as snakes, hawks, and wolves, which are then decomposed by scavengers and decomposers such as bacteria and fungi. These decomposers break down the remains of dead organisms and return nutrients to the soil, where they can be taken up by plants like grasses to start the cycle anew.
Therefore, grasses are a critical component of the food web, serving as the foundation for energy flow and supporting the survival of many different species in the ecosystem.
To learn more about food web follow the given link: https://brainly.com/question/15544799
#SPJ1
The heat of vaporization delta Hv of dichloromethane (Ch2CL2) is 28.0 kJ/mol . Calculate the change in entropy delta S when 473 g of dichloromethane boils at 39.8 degree.
Answers
Answer:
16 J/K.mol
Explanation:
From the question,
ΔS = ΔH/T............... Equation 1
Where ΔH = Heat change, T = Temperature
But,
ΔH = n(Hv).................. Equation 2
Where n = number of mole, Hv = heat of vaporization.
Given: Hv = 28.0 kJ/mol, n = 473/85 = 5.59 mole.
Substitute these values into equation 2
ΔH = 28/5.59
ΔH = 5.01 kJ.
Also: T = 273+39.8 = 312.8 J
Substitute into equation 1
ΔS = 5.01/312.8
ΔS = 0.016 kJ/K
ΔS = 16 J/K.mol
You are in a laboratory creating a new chocolate bar. You want to create the sweetest chocolate bar by maximizing the sugar concentration. You are doing this by adding the sugar to a chocolate mixture. Which would allow you to dissolve more sugar?
The answer: Add the sugar after heating the mixture.
Answers
Adding the sugar after heating the mixture would allow you to dissolve more sugar, which would result in a sweeter chocolate bar.
When you dissolve sugar in a liquid, such as in a chocolate mixture, there is a limit to the amount of sugar that can be dissolved at a given temperature. This limit is known as the solubility of the sugar in that liquid. The solubility of sugar in water is higher at higher temperatures, which means that you can dissolve more sugar in hot water than in cold water. The same principle applies to chocolate mixtures.
By heating the chocolate mixture, you increase the temperature of the mixture, which in turn increases the solubility of the sugar in the mixture. This allows you to dissolve more sugar in the mixture than if you were to add the sugar to the mixture at room temperature or when it is cold.
To know more about solubility of sugar here
https://brainly.com/question/2279296
#SPJ1
--The given question is incorrect, the correct question is
"You are in a laboratory creating a new chocolate bar. You want to create the sweetest chocolate bar by maximizing the sugar concentration. You are doing this by adding the sugar to a chocolate mixture. Which would allow you to dissolve more sugar?"--
30 example of redox reaction
Answers
1. Combustion of gasoline in a car engine
2. Rusting of iron
3. Photosynthesis in plants
4. Respiration in animals
5. Corrosion of metals
6. Bleaching of hair with hydrogen peroxide
7. Formation of ozone in the atmosphere
8. Electroplating of metals
9. Burning of wood
10. Reaction between bleach and ammonia
11. Reaction between copper and nitric acid
12. Reaction between iron and hydrochloric acid
13. Reaction between zinc and sulfuric acid
14. Reaction between magnesium and hydrochloric acid
15. Reaction between aluminum and hydrochloric acid
16. Reaction between sodium and water
17. Reaction between potassium and water
18. Reaction between lithium and water
19. Reaction between calcium and water
20. Reaction between barium and water
21. Reaction between copper and silver nitrate
22. Reaction between lead and silver nitrate
23. Reaction between zinc and copper sulfate
24. Reaction between iron and copper sulfate
25. Reaction between magnesium and copper sulfate
26. Reaction between aluminum and copper sulfate
27. Reaction between sodium and chlorine
28. Reaction between magnesium and chlorine
29. Reaction between aluminum and chlorine
30. Reaction between zinc and hydrochloric acid.
A calorie is a unit of measurement that measures what?
Answers
Answer:
Okay
Explanation:
45.57 mL of a solution is diluted to 63.40 mL. The diluted solution is found to have a concentration of 0.433 N. What was the concentration of the original solution?
Answers
The correct answer is 0.55 M
Given,
Volume of solution before diluting = 45.45 ml
Volume of solution after diluting = 63.4 ml.
Concentration of diluted solution = 0.4 M
Concentration of a solution is referred to the amount of water present in the solution.
To answer this problem we can use the following formula:
V₁C₁ = V₂C₂
Where the subscript 1 refers to the volume and concentration before diluting, and 2 refers to volume and concentration after diluting.
Meaning that in this case we can write:
45.57 mL * C₁ = 63.4 mL * 0.4 M
We can now solve for C₁:
C₁ = 0.55 M
Therefore the concentration of original solution is 0.55 M.
To know more about concentration, refer: https://brainly.com/question/12402373
#SPJ9
explain why d-block and transition metal should not be used interchangeably ?
Answers
Answer:
The d-block and transition metal are not interchangeable terms because the d-block elements are a subset of the transition metal elements. The transition metals are defined as the elements that have partially filled d orbitals, which includes the d-block elements as well as other elements that have partially filled d orbitals in other blocks, such as lanthanides and actinides. Therefore, while all d-block elements are transition metals, not all transition metals are d-block elements.
State three precautions necessary to ation. explain how you can prepare 0.2m solution of tetraoxosulphate (VI) acid in 400cm³ volumetric flask. (CH=1, 0=16, S=32; specify gravity = 1.84 percentage purity=98) Halls
Answers
Wear personal defence tools, follow the guidelines and be careful with chemicals. Measure 14.72g of \(H_2SO_4\), dissolve in distilled water, and make up to 400mL in a volumetric flask.
1. Always wear appropriate personal protective equipment such as gloves, goggles, and lab coat.
2. Read and follow the instructions carefully before handling any chemical.
3. Handle the chemicals in a well-ventilated area to prevent inhalation of harmful fumes.
To prepare a 0.2M solution of tetraoxosulphate (VI) acid in a \(400cm^3\) volumetric flask:
Calculate the amount of tetraoxosulphate (VI) acid required using the formula:
Mass = (Molarity x Volume x Molecular weight) / 1000
Where:
Molarity = 0.2M
Volume =\(400cm^3\)
Molecular weight = (4x16) + 32 + (6x16) = 98g/mol
Mass = (0.2 x 400 x 98) / 1000 = 7.84g
Weigh out 7.84g of tetraoxosulphate (VI) acid using a balance.
Transfer the weighed tetraoxosulphate (VI) acid into the \(400cm^3\) volumetric flask using a funnel.
Add distilled water to the flask until the volume reaches the \(400cm^3\) mark on the neck of the flask.
Stopper the flask and mix the solution thoroughly by inverting the flask several times.
It is important to specify the density of the tetraoxosulphate (VI) acid, as this will affect the mass required for the solution. In this case, the percentage purity of the acid is also given, which can be used to calculate the actual mass of the tetraoxosulphate (VI) acid needed.
For more such questions on chemicals, click on:
https://brainly.com/question/28404194
#SPJ11
Dimensional analysis with shapes

Answers
The surface area of the rectangular prism is 0.034 square meters.
For a rectangular prism with length l, width w, and height h, the surface area is:
Surface area = 2lw + 2lh + 2wh
Substituting the given values, we get:
Surface area = 2(10 cm x 5 cm) + 2(10 cm x 8 cm) + 2(5 cm x 8 cm)
Surface area = 100 cm² + 160 cm² + 80 cm² = 340 cm²
We can use dimensional analysis. So the conversion factor is:
1 m² / 10,000 cm²
Multiplying the surface area by this conversion factor, we get:
Surface area = 340 cm² x (1 m² / 10,000 cm²)
Surface area = 0.034 m²
To know more about rectangular prism, here
brainly.com/question/21308574
#SPJ1
--The complete Question is, What is the surface area of a rectangular prism that has a length of 10 cm, a width of 5 cm, and a height of 8 cm? Use dimensional analysis to convert the answer to square meters--
Combustion of an unknown compound containing only carbon and hydrogen produces 12.3 g of CO₂ and 3.8 g of H₂O. What is the empirical formula of the compound?
Answers
Answer:
C2H3
Explanation:
.
The empirical formula of the compound is C3H3.
What is an empirical formula?
In science, the exact equation of a synthetic compound is the most straightforward entire number proportion of particles present in a compound. A basic illustration of this idea is that the experimental recipe of sulfur monoxide, or somewhere in the vicinity, would just be Thus, similar to the observational recipe of di sulfur dioxide, S2O2.
In this manner, sulfur monoxide and di sulfur dioxide, the two mixtures of sulfur and oxygen, have a similar exact equation. Nonetheless, their sub-atomic equations, which express the quantity of iotas in every particle of a synthetic compound, are not something similar.
Learn more about empirical formula here:
https://brainly.com/question/11588623
#SPJ2
Part A
Review | Constants | Periodic Tab
What volume of 0.205 M K3PO4 solution is necessary to completely react with 114 mL of 0.0118 M NiCl
Express your answer to three significant figures.
Reaction
2 K3PO4 (at) + 3 NiCl2 (aq) arrow Ni3 (PO4)2(s) +6KCl (aq)
Answers
The volume of 0.205 M K3PO4 solution necessary to completely react with 114 mL of 0.0118 M NiCl2 solution is 0.00437 L or 4.37 mL
The given chemical equation shows that two moles of K3PO4 react with three moles of NiCl2 to form one mole of Ni3(PO4)2 and six moles of KCl. Thus, the stoichiometric ratio of K3PO4 to NiCl2 is 2:3.
To calculate the volume of K3PO4 solution required to completely react with 114 mL of 0.0118 M NiCl2 solution, we need to use the concept of stoichiometry and the equation of concentration, C = n/V, where C is the concentration in moles per liter (M), n is the amount in moles, and V is the volume in liters.
First, we can calculate the amount of NiCl2 in 114 mL of 0.0118 M solution:
n(NiCl2) = C × V = 0.0118 M × 0.114 L = 0.0013452 mol
Next, we can use the stoichiometric ratio to calculate the amount of K3PO4 required:
n(K3PO4) = (2/3) × n(NiCl2) = (2/3) × 0.0013452 mol = 0.0008968 mol
Finally, we can use the equation of concentration to calculate the volume of 0.205 M K3PO4 solution required:
V(K3PO4) = n(K3PO4) / C(K3PO4) = 0.0008968 mol / 0.205 M = 0.00437 L
Therefore, the volume of 0.205 M K3PO4 solution necessary to completely react with 114 mL of 0.0118 M NiCl2 solution is 0.00437 L or 4.37 mL (to three significant figures).
For more such questions on volume
https://brainly.com/question/29796637
#SPJ11
The mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produced 23.5 g of sodium upon decomposition. How much fluorine was formed?
Answers
19.42g of fluorine is produced upon decomposition of sodium fluoride.
What is mass ratio?The mass of a given substance is converted to moles using the molar mass of this substance in the periodic table. Moles of a given substance are then converted to moles of an unknown substance using the molar ratios from the balanced chemical formulas.
Mass ratio is defined as the percentage composition of the masses of elements in a molecule or compound. A compound always has a defined mass fraction of the corresponding element.
Mass ratio of sodium to fluorine = 1.21:1
If the mass of sodium fluoride produced is 23.5 g
Using dimensional Analysis,
(23.5g of sodium/sodium fluoride)×(1 g of Fluorine/1.21 g of sodium)
= 19.42g(g of fluorine/g of sodium fluoride)
Mass of fluorine produced = 19.42g
To know more about mass ratio, visit:
https://brainly.com/question/14561456
#SPJ1
what is the mass in grams of 1.71 x 10^34 molecules of ketamine?
Answers
The mass of ketamine is 66.56 ×\(10^{11}\) g.
Number of mole = Avogadro number / number of molecules
Avogadro number = 6.02 × \(10^{23}\)
Number of molecules = 1.71 ×\(10^{34}\)
Number of mole = 1.71 ×\(10^{34}\) / 6.02 × \(10^{23}\) = 0.28 ×\(10^{11}\)
Number of mole = given mass / molar mass
Molar mass of ketamine = 237.72 g
Number of mole = 0.28 ×\(10^{11}\)
Hence, given mass = Number of mole × molar mass
Given mass = 0.28 ×\(10^{11}\) × 237.72 g
Given mass = 66.56 ×\(10^{11}\) g
So, the mass of ketamine is 66.56 ×\(10^{11}\) g.
Avogadro number is number 6.022 × 10²³ is known as Avogadro's number or Avogadro's constant. The concept of the mole can be used to convert between mass and number of particles.
Learn more about Avogadro number here:- https://brainly.com/question/14138110
#SPJ1
If 32.0 grams of ammonium nitrite react to form 14.0 grams of nitrogen, how many grams of water must simultaneously be formed?
Answers
The mass of water that is formed is 20 grams of water.
What is decomposition?A decomposition reaction is one in which a substance is broken down to give its components. The decomposition would lead to the formation of the substances that were combined to obtain the decomposed substance.
From the law of conservation of mass, we know that the total mass in the system does not change. This implies that the mass of the reactants must be equal to the mass of the products.
If the mass of the ammonium nitrite is 34 grams then the mass of the water that is formed simultaneously is; 34 - 14 = 20 grams of water.
Learn more about conservation of mass:https://brainly.com/question/13383562
#SPJ1
1H
35Cl has a force constant (k) value of 480 Nm-1
. Calculate the fundamental frequency
and its wavenumber.
Answers
The wave number of the compound can be calculated as shown and we are going to have 8.651 x 10^13 s^-1.
What is the force constant of molecule?The force constant of a molecule is a measure of the strength of the chemical bonds between the atoms in the molecule. It is defined as the second derivative of the potential energy of the molecule with respect to atomic displacements, evaluated at the equilibrium geometry of the molecule.
μH35Cl = 1.627 x 10^-27 kg
v = 1/2π x (480.5kg m s^-2 / 1.627 x 10^-27 kg)^1/2
v = 0.1592 x (2.953 x 10^29 m s^-2)^1/2
v = 0.1592 x (5.434 x 10^14 s^-1)
v = 8.651 x 10^13 s^-1
Learn more about force constant:https://brainly.com/question/30951247
#SPJ1
what is the mass of 1mol of chlorine ions?
Answers
Answer:
71g
Explanation:
i think thats right?
One mole of chlorine would be 71 g unless they said "1 mole of chlorine ATOMS".
HELP HELP !!
All stimuli must be sent to ur brain for a response to occur
true or false?
Answers
. A popular model for the
was that it was static: It is and always has been as we see it now, and in general hasn’t changed.
Answers
A popular model for the nucleus of an atom was that it was static. It is and always has been as we see it now, and in general hasn’t changed.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Learn more about atom,here:
https://brainly.com/question/29695801
#SPJ1
Jamal is working with three ionic compounds: sodium chloride, calcium sulfide, and barium oxide. His teacher asks him which are the positive ions in each compound. What should Jamal’s answer be? sodium, calcium, and barium barium, sulfur, and oxygen calcium, barium, and chlorine chlorine, sulfur, and oxygen
Answers
Answer:
option A is correct ( sodium, calcium and barium)
Explanation:
Given compounds:
Sodium chloride , calcium sulfide, barium oxide
We know that metals form positive ions. In order to solve the problem we must identify the metals from given compounds.
Na⁺Cl⁻
Ca²⁺S²⁻
Ba²⁺O²⁻
We can see that sodium, calcium and barium contain positive charges.
Thus option A is correct.
Because sodium have one valance electron. When it combine with chlorine sodium lose its one electron to complete the octet and chlorine accept it to complete its octet. Thus sodium form positive ion and chlorine form negative ion.
Similarly barium and calcium are present in group 2. Both have two valance electron. When they lose them cation are formed.
Other option are incorrect because,
Option B have sulfur and oxygen which are anion.
Option C have chlorine which is also anion
Option D have chlorine, sulfur and oxygen that are anions.
Answer:
option A is correct ( sodium, calcium and barium)
Explanation:
What mass (grams) of sodium sulfate would be formed by the complete reaction of 120.0 grams of sodium hydroxide?
Answers
Answer:
The mass of sodium sulfate formed by the comolete reaction of 120.0 grams of sodium hydroxide is 142.04 grams.
Explanation:
The balanced chemical equation for the reaction between sodium hydroxide and sulfuric acid is:
2NaOH + H2SO4 -> Na2SO4 + 2H2O
From the equation, we can see that 2 moles of sodium hydroxide react with 1 mole of sulfuric acid to form 1 mole of sodium sulfate. We can use this information, along with the molar masses of the compounds, to calculate the mass of sodium sulfate formed.
First, we need to convert the given mqss of sodium hydroxide to moles. The molar mass of sodium hydroxide is 40.00 g/mol, so:
Moles of NaOH = Mass of NaOH / Molar mass of NaOH
Moles of NaOH = 120.0 g / 40.00 g/mol
Moles of NaOH = 3.00 mol
Next, we can use the mole ratio from the balanced equation to calculate the moles of sodium sulfate formed:
Moles of Na2SO4 = Moles of NaOH / 2
Moles of Na2SO4 = 3.00 mol / 2
Moles of Na2SO4 = 1.50 mol
Finally, we can convert the moles of sodium sulfate to grams using its molqr mass of 142.04 g/mol:
Mass of Na2SO4 = Moles of Na2SO4 x Molar mass of Na2SO4
Mass of Na2SO4 = 1.50 mol x 142.04 g/mol
Mass of Na2SO4 = 213.06 g
Therefore, the mass of sodium sulfate formed by the complete reaction of 120.0 grams of sodium hydroxide is 213.06 grams.
If 3.0 grams of Strontium-90 in a rock sample remained in 1989, approximately how many grams of Strontium-90 were present in the original sample in 1933?
Answers
11.29 grams of strontium-ninety had been gift withinside the unique rock pattern in 1933. Strontium- ninety decays to yttrium-ninety, which in flip decays to solid zirconium.
The isotopes of strontium and yttrium emit beta debris as they decay. The launch of radiation throughout this decay manner reasons situation approximately the protection of strontium and all different radioactive substances. Sr-ninety may be inhaled, however ingestion in meals and water is the best fitness situation. Once withinside the body, Sr-ninety acts like calcium and is effectively included into bones and teeth, wherein it may purpose cancers of the bone, bone marrow, and smooth tissues across the bone.
Strontium has a half-existence of 28.8 years. Therefore,
1989 - 1933 = 56years.
fifty six / 28.eight = 1.94 half-lives
Thus, the amount of the radioisotope closing will double for every half-existence elapsed. transferring backwards in time. Therefore, transferring backwards in time through 1.94 times half-lives, the amount closing will double through 1.94 times.
Thus, the quantity closing in 1933 is 3.0 × (1.ninety four)² = 11.29 grams.
Learn more about Strontium-90 visit: brainly.com/question/14351021
#SPJ4
]All organic compounds contain the element carbon but, not all compounds containing the element “carbon”are organic .Justify this statement.
Answers
The statement "All organic compounds contain the element carbon, but not all compounds containing the element 'carbon' are organic" can be justified based on the definition and characteristics of organic compounds.
Organic compounds are compounds primarily composed of carbon and hydrogen atoms, often with other elements like oxygen, nitrogen, sulfur, and phosphorus. These compounds are typically associated with living organisms and are known for their unique properties and behavior, including the ability to form complex structures, exhibit covalent bonding, and undergo organic reactions.
On the other hand, there are compounds that contain carbon but are not classified as organic. One notable example is carbon dioxide (\(CO_{2}\)), which is a simple inorganic compound composed of carbon and oxygen. Carbon dioxide does not possess the characteristic properties of organic compounds, such as the ability to form long chains or undergo organic reactions.
Additionally, there are inorganic compounds like carbonates (such as calcium carbonate) and carbides (such as calcium carbide) that contain carbon but are not considered organic. These compounds have distinct chemical and physical properties different from those of organic compounds.
In summary, while all organic compounds contain carbon, not all compounds containing carbon are organic. The classification of a compound as organic or inorganic depends on its overall molecular structure, bonding, and characteristic properties.
Know more about molecular structure here:
https://brainly.com/question/27789666
#SPJ8
