Answers
Answer:
It adds new evidence
Explanation:
I just took them exam
Related Questions
part 1 of 5
A 155 mL flask contains argon at 1.4 atm
and 86°C. What amount of Ar is present?
Answer in units of mol.
Answers
Considering the definition of ideal gas law, the amount of Ar present is 0.00702 moles.
Definition of ideal gas law.An ideal gas is a set of atoms or molecules that move freely without interactions. The pressure exerted by the gas is due to the collisions of the molecules with the walls of the container. Ideal gas behavior occurs at low pressures. At high pressures the molecules interact and intermolecular forces cause the gas to deviate from ideality.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P×V = n×R×T
Where:
P is the gas pressure.V is the volume that occupies.T is its temperature.R is the ideal gas constant. The universal constant of ideal gases R has the same value for all gaseous substances. n is the number of moles of the gas. Amount of ArIn this case, you know:
P= 1.4 atmV= 155 mL= 0.155 L (being 1000 mL= 1 L)T= 86 °C= 359 K (being 0 C= 273 K)R= 0.086 (atmL)÷(molK)n= ?Replacing in the definition of ideal gas law:
1.4 atm× 0.155 L = n×0.086 (atmL)÷(molK)× 359 K
Solving:
[1.4 atm× 0.155 L] ÷ [0.086 (atmL)÷(molK)× 359 K]= n
0.00702 moles= n
Finally, there are 0.00702 moles of Ar.
Learn more about ideal gas law:
https://brainly.com/question/4147359
#SPJ1
How do particles tend to move in solids?
They vibrate about fixed points.
They move randomly through the solid while remaining in close contact with other particles.
They move in orbits around a center.
They move randomly through the solid and separately from other particles.
Answers
Please i meed help quick and thank you

Answers
It is the 4th scenario is the dependent event. There are 7 gold tokens and 4 silver tokens in a cup. The first student randomly draws a gold token and keeps it. A second student randomly draws a gold token from the cup.
How did we identify the dependent event?The fouth scenario is a dependent event because the probability of the second student drawing a gold token is affected by the outcome of the first student's draw.
If the first student draws a gold token, then there are only 6 gold tokens left in the cup, the probability changes. but if the first student does not draw a gold token, then there are 7 gold tokens left in the cup, the probability will remain the same
Find more exercises on dependent events;
https://brainly.com/question/11473170
#SPJ1
A 0.4042 g sample of a pure soluble bromide compound is dissolved in water, and all of the
bromide ion is precipitated as AgBr by the addition of an excess of silver nitrate. The mass of
the resulting AgBr is found to be 0.6797 g.
What is the mass percentage of bromine in the original compound?
%
Answers
70.80 % is the mass percentage of bromine in the original compound.
Mass of AgBr = 0.6797 g
Moles of AgBr = 0.6797 g / 187.77 g/mol
= 0.0036198 mol
Silver Nitrate + Bromine ---------> Silver Bromide + Nitrate
According to reaction, 1 mole of AgBr is obtained from 1 mole of bromide ions , then 0.0036198 moles of AgBr will be formed from :
1/1 * 0.0036198 mol = 0.0036198 mole of bromide ions
Mass of 0.0036198 moles of bromide ions :
0.0036198 moles * 79.09 g/ mol
= 0.2862 g
Mass of the sample = 0.4042 g
Mass percentage = 0.2862 * 100 / 0.4041
Mass percentage (%) = 70.80 %
Hence, the mass percentage of bromine in the original compound is 70.80%
To know more about mass percentage here :
https://brainly.com/question/16885872?referrer=searchResults
#SPJ1
A type of emergency apparatus that can be used where oxygen may be limited or where the air might be poisoned is based on the following reaction in which CO2 produced by your own respiration reacts and O2 gas is produced. Calculate the mass of KO2 needed to produce 25 g of oxygen gas.
4 KO2(s) + 2 CO2(g) 2 K2CO3(s) + 3 O2 (g)
Answers
Answer:
so I don't know how to answer that
Which statements are true about balancing chemical equations?
Check all that apply.
A. Balancing chemical equations does not involve trial and error.
B. Balancing chemical equations involves trial and error.
C. Atoms that are in only one of the reactants and only one of the
products should be done last.
D. Single atoms should be done last.
Answers
Answer:
statement C is the correct answer
ca(s) zncl then, write balanced half-reactions describing feso4 mg the oxidation and reduction that happen in this reaction g
Answers
The calcium molecule undergoes oxidation while zinc molecule undergoes reduction during the redox reaction.
What is redox ?
It is a type of reaction in which there is an exchange of electron between two reactant species. During which one reactant under reduction and the other reactant undergoes oxidation.
Let the atoms in it ionic form-
\(Ca (s) + Zn^{2+} (aq)\) → \(Zn (s) + Ca^{2+} (aq)\)
Oxidation :
\(Ca (s)\) → \(Ca^{2+} (aq) + 2e^{-}\)
Reduction :
\(Zn^{2+} (aq) + 2e^{-}\) → \(Zn (s)\)
Hence we can see that in the following redox reaction, calcium changes its oxidation state from 0 to +2 and zinc changes its oxidation state from +2 to 0 as it undergoes reduction.
To learn more about redox click here:
https://brainly.com/question/459488
#SPJ4
What is the boiling point in °C of a 3.6 molal solution of calcium chloride in water?

Answers
Answer:
CaCl2--->Ca2+ + 2Cl_ so i=3
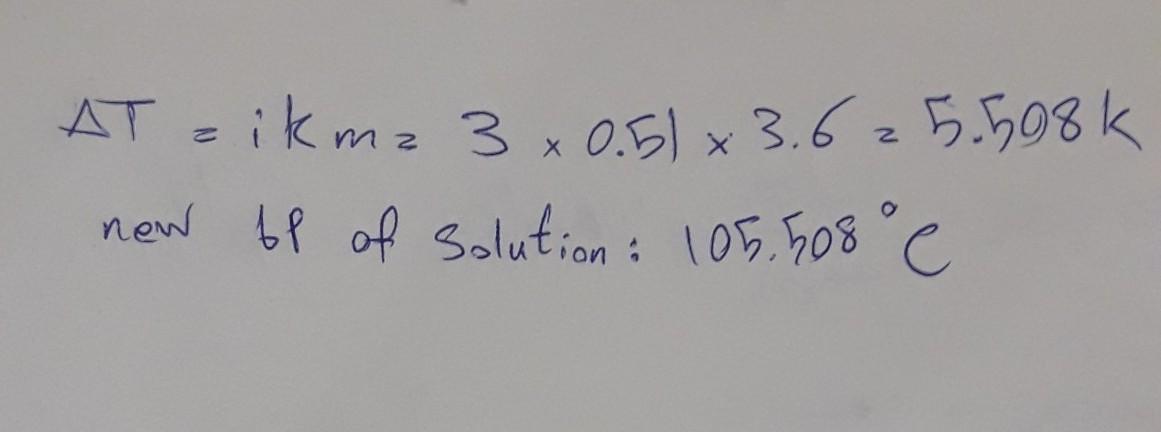
16. A sequence of star colors frorn hottest to coolest is
O f. blue, yellow, orange, red
g. red, orange, yellow, blue
0 h. blue, red, yellow, orange
1 yellow, blue, orange, red
Answers
Answer:
g. red, orange, yellow, blue
Explanation:
red is the hottest color and blue is the coolest. :)
scientist wants to use a model to help present the results of his detailed scientific investigation.
Why would a model be useful?
because the model makes the concepts easier to understand
because the model is easy to put together and to use
because the model prevents other scientists from asking questions
because the model requires the audience to pay full attention to it
Answers
Answer: A model would be useful because the model makes the concepts easier to understand.
Explanation:
Models are helpful tools in science education that can be used to enhance explanations, spark discussion, make predictions, provide visual representations of abstract concepts, and create mental models.
The idea that the moon, sun, and known planets orbit Earth is called the _______________ model of the universe.
Answers
Answer:
spherical model of the universe
Heliocentric model- planetary bodies orbit the Sun
1. in this experiment, why 3-sulfolene was used instead of 1,3-butadiene? explain thoroughly for full credit.
Answers
Starting with solid 3-sulfolene and then breaking it down was simpler than doing it with gaseous 1,3-butadiene. Maleic anhydride, a dienophile, reacts with the diene to produce 4-cyclohexene-cis-dicarboxylic anhydride.
What is sulfolene ?A cyclic organic compound with a sulfone functional group is known as sulfolene or butadiene sulfone. It is a crystalline, odorless, white solid that can be stored forever and dissolves in various organic solvents as well as water. The substance is utilized as a butadiene source.
Sulfolane is a common industrial solvent that is used for cleaning natural gas and extracting aromatic hydrocarbons from hydrocarbon mixtures.
Sulfolane, a dipolar aprotic sulfone solvent, is comparable in physicochemical qualities to other dipolar aprotic solvents as DMSO, NMP, DMF, and DMAC. Sulfolane (anhydrous) has the highest freezing point and highest boiling point among the solvents in Table 1 at 28.4 °C.
Thus, solid 3-sulfolene and then breaking it down was simpler than doing it with gaseous 1,3-butadiene.
To learn more about sulfolene, follow the link;
https://brainly.com/question/29854277
#SPJ1
1. Answer the following questions regarding the peptide LCYRAIDCG a) What is the sequence of amino acids written as the THREE letter code? b) Draw the structure of the peptide LCYRAIDCG as it would appear at pH 5 under oxidizing conditions. Be sure to include any disulfide bonds that would form. c) Label the N-terminus of the peptide in b) with a tick (√) d) Label the C-terminus of the peptide you drew in b) with a star (*)
Answers
b) Drawing the structure of the peptide LCYRAIDCG at pH 5 under oxidizing conditions with disulfide bonds:
H3N+ - Leu - Cys(S-S)- Tyr - Arg - Ala - Ile - Asp - Cys(S-S)- Gly - COO-
c) The N-terminus of the peptide in b) would be labeled with a tick (√) at the amino group (H3N+).
d) The C-terminus of the peptide in b) would be labeled with a star (*) at the carboxyl group (COO-).
describe what xeriscaping is and what is involved in a successful xeriscaping project
Answers
Xeriscaping is a landscaping approach that focuses on conserving water by using drought-tolerant plants and efficient irrigation techniques. The goal is to create a visually appealing and sustainable garden while minimizing water usage.
Successful xeriscaping projects involve several key elements. Firstly, careful plant selection is crucial, opting for species that can thrive in arid conditions without excessive watering. Mulching is used to reduce evaporation and retain soil moisture.
Proper soil preparation, such as improving drainage and adding organic matter, promotes healthier plant growth. Efficient irrigation systems, like drip irrigation or soaker hoses, deliver water directly to plant roots, minimizing wastage.
Additionally, controlling erosion through the use of retaining walls or terracing is important. Lastly, regular maintenance, including appropriate pruning and weed control, ensures the longevity and vitality of the xeriscape garden. Overall, a successful xeriscaping project harmonizes sustainable practices with a beautiful outdoor environment.
For more such questions on Xeriscaping
https://brainly.com/question/12960529
#SPJ11
true/false. label the functional groups in the molecule. you are currently in a labeling module. turn off browse mode or quick nav, tab to items, space or enter to pick up, tab to move, space or enter to drop.a molecule is composed of several functional groups.
Answers
The statement is true that a molecule is composed of several functional groups.
What is molecules?A molecule is a group of two or more atoms held together by chemical bonds. Atoms can form chemical bonds by sharing electrons with other atoms, leading to the formation of stable molecules with unique chemical and physical properties. Molecules can be made up of atoms of the same element or different elements, and they can vary widely in size and complexity. In biology, many important molecules are made up of carbon, hydrogen, oxygen, nitrogen, and other elements, and they play critical roles in biological processes such as metabolism, cellular signaling, and genetic information storage and transmission. Examples of important biological molecules include DNA, RNA, proteins, carbohydrates, lipids, and many others. These molecules are responsible for carrying out the various functions of cells and organisms and are critical for life as we know it.
Here,
Many molecules in biology are composed of several functional groups, which are specific atoms or groups of atoms within the molecule that give it its chemical properties and reactivity. Examples of common functional groups in biological molecules include amino (-NH2), carboxyl (-COOH), hydroxyl (-OH), phosphate (-PO4), and methyl (-CH3) groups, among others. The presence of these functional groups can determine how a molecule interacts with other molecules in the cell and can influence its function and activity.
To know more about molecules,
https://brainly.com/question/11932695
#SPJ1
A 50.00mL sample of solution containing Fe^2+ ions is titrated with a 0.0216 M KMnO4 solution. It required 20.62mL of KMnO4 solution to oxidize all the Fe^2+ ions to Fe^3+ ions by reaction.
MnO4- (aq) + Fe^2+ (aq) ------> Mn^2+ (aq) + Fe^3+ (aq) (unbalanced)
acidic
a.) What was the concentration of Fe^2+ ions in the sample solution?
b.) What volume of 0.0150 M K2Cr2O7 solution would it take to do the same titration? The reaction is
Cr2O7^2- (aq) + Fe^2+ (aq) ---------> Cr^3+ (aq) + Fe^3+ (aq) (unbalanced)
acidic
Answers
The concentration of Fe\(^+2\) ions is 0.0088 M, and 29.33 L of K₂Cr₂O₇ is required for the titration.
How to calculate concentration of solution?Concentration of a titrand when volume is given can be calculated as,
M₁V₁=M₂V₂
Likewise volume of titrand can also be calculated by the same formula.
In the given problem, concentration of KMnO₄ =0.0216 M and it's volume=20.62 ml or 0.0206 L, volume of Fe\(^+2\) ions=50 ml or 0.05 L
substituting the values in the formula,
M₁=0.0216×0.0206/0.05=0.0088 M.
For calculating the volume of potassium dichromate using the same formula ,
V₂=0.0088×0.05/0.015=0.02933 ml or 29.33 L.
Thus, the concentration of iron(II) ions in sample is 0.0088 M and 29.33 L of 0.015 M potassium dichromate is used for doing the same titration.
To know more about molar concentrations click here:
https://brainly.com/question/21841645
#SPJ1
burning 12g of urea raise temp of water by 30C what is the enthalpy of combustion for 1kg urea
Answers
The enthalpy of combustion for 1kg of urea is -1223525.84 J/mol.
Urea is a compound that is used in fertilizers and in some plastics.The enthalpy of combustion for urea is the amount of energy that is released when urea is burned. In order to calculate the enthalpy of combustion for 1kg of urea, we need to use the information that is provided to us in the question. Let us start by writing down the balanced equation for the combustion of urea: CO(NH2)2 + 3/2 O2 → CO2 + 2H2O + N2
The balanced equation shows that 1 mole of urea reacts with 1.5 moles of oxygen gas to produce 1 mole of carbon dioxide, 2 moles of water, and 1 mole of nitrogen gas. The enthalpy change for this reaction is equal to the amount of energy that is released when 1 mole of urea is burned.
The heat of combustion (ΔHc) of urea is -632.6 kJ/mol. This means that 632.6 kJ of energy is released when 1 mole of urea is burned. We know that 12g of urea raised the temperature of water by 30°C. We can use this information to calculate the amount of energy that was released when 12g of urea was burned.
The specific heat capacity of water is 4.18 J/g°C. This means that it takes 4.18 J of energy to raise the temperature of 1 gram of water by 1°C. Therefore, it takes 4.18 x 1000 = 4180 J of energy to raise the temperature of 1 kg of water by 1°C.
We know that 12g of urea raised the temperature of water by 30°C. Therefore, the amount of energy that was released when 12g of urea was burned is:
Energy = mass x specific heat capacity x temperature change
Energy = 0.012 kg x 4180 J/kg°C x 30°C
Energy = 1497.6 J
We can now use this information to calculate the enthalpy of combustion for 1kg of urea:
Enthalpy of combustion = energy released / moles of urea burned
Enthalpy of combustion = 1497.6 J / (0.012 kg / 60.06 g/mol)
Enthalpy of combustion = - 1223525.84 J/mol
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
D » » DI
Which applies to fusion? Check all that apply.
involves the splitting of nuclei
takes place in the Sun
releaſes radiation as a waste product
occurs in nuclear power plants and is used to generate electricity
plays a role in the production of essentially all elements heavier than helium
releases large amounts of energy
I’m looking for fusion not fission
Answers
Answer:
The correct answer is -
takes place in the Sun
plays a role in the production of essentially all elements heavier than helium
releases large amounts of energy
Explanation:
Fusion reaction takes place when two or more small atomic nuclei come in close proximation for a longer time so the nuclear force pulling them together and form into heavier molecules than helium and releases a huge amount of energy by this process.
A great example of this fusion reaction is the sun where nuclear fusion takes place inside the core of the sun and result in a huge amount of release as it is an exothermic reaction.
Answer:
2, 5, 6Explanation
EDGE2021
write all reaction of phenols in the following
Answers
Answer:
Hope this is what you were looking for.
Phenol reacts with dilute nitric acid at room temperature to give a mixture of 2-nitrophenol and 4-nitrophenol while with concentrated nitric acid, more nitro groups get substituted on the ring to give 2,4,6-trinitrophenol which is known as picric acid.
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
If you had 6H2 molecules and 4O2 molecules, how many H2O molecules could you produce?
Answers
Answer:
6
Explanation:
As , 2H2 + O2 = 2H2O
with 6 H2, 4O2 is excess.
H2O molecules formed = 6
In Part A, we saw that the theoretical yield of aluminum oxide is 1.60 mol . Calculate the percent yield if the actual yield of aluminum oxide is 1.22 mol .
Answers
Taking into account definition of percent yield, the percent yield for the reaction is 76.25%.
Percent yieldThe percent yield is the ratio of the actual return to the theoretical return expressed as a percentage.
The percent yield is calculated as the experimental yield divided by the theoretical yield multiplied by 100%:
\(percent yield=\frac{actual yield}{theorical yield}x100\)
where the theoretical yield is the amount of product acquired through the complete conversion of all reagents in the final product, that is, it is the maximum amount of product that could be formed from the given amounts of reagents.
Percent yield in this caseIn this case, you know:
actual yield= 1.22 moltheorical yield= 1.60 molReplacing in the definition of percent yields:
\(percent yield=\frac{1.22 mol}{1.60 mol}x100\)
Solving:
percent yield= 76.25%
Finally, the percent yield for the reaction is 76.25%.
Learn more about percent yield:
brainly.com/question/14408642
#SPJ1
look at the reaction below and state which direction the reaction would shift:
2Hg0 <=> 2Hg +O2
Answers
Answer:
there is no shift in the state
Explanation:
The correct answer is - There is no shift in the state.
Reason -
If K > Q, a reaction will proceed forward, converting reactants into products. If K < Q, the reaction will proceed in the reverse direction, converting products into reactants. If Q = K then the system is already at equilibrium.
where Q, is the reaction Quotient
6. Which scientist said it was impossible to know both the location and momentum of an electron?a. Bohrb. ThomsonC. Rutherfordd. Heisenberg
Answers
The uncertainty principle was discovered by Heisenberg. It states that ΔpΔx ≥ h where Δp is the uncertainty in knowing the momentum of the particle (momentum equals mass times velocity), Δx is the uncertainty in knowing the position of the particle, and h is Planck's constant (h=6.63×10⁻ ³⁴Js). If we know very precisely the position of the particle, we will not be able to know its speed so precisely and vice versa, regardless of how good our measuring device is.
So the answer is option d)
URGENT!!! An unknown hydrate of CoCl₂ has been evaporated in a crucible. Given the following data, find the formula and name of the hydrate.
Mass of crucible: 12.090 g
Mass of hydrate before evaporation and crucible: 16.250 g
Mass of hydrate after evaporation and crucible: 12.424 g
Answers
From the given data, the name of the hydrated salt would be \(CoCl_2.83H_2O\).
Formula of hydrateThe formula of the hydrated salt can be determined using the empirical formula approach. That is, we will find the mole equivalent of the anhydrous salt and the water of hydration and then combine them into a single formula after dividing by the smallest mole.
First, we need to determine the mass of the anhydrous salt and the water of hydration.
Mass of crucible (x) = 12.090 g
Mass of hydrated salt + crucible (y) = 16.250 g
Thus, the mass of the hydrated salt can be determined by subtracting x from y.
Mass of hydrated salt = 16.250 - 12.090 = 4.16 g
Mass of hydrate + crucible after evaporating off the water (z) = 12.424 g
Mass of anhydrous salt = z - x
= 12.424 - 12.090
= 0.334 g
Mass of water = 4.16 - 0.334
= 3.826 g
Now, let's find the moles:
Molar mass of \(CoCl_2\) = 129.839 g/mol
Molar mass of water = 18.01 g/mol
Mole of \(CoCl_2\) = 0.334/129.839 = 0.00257 mol
Mole of water = 3.826/18.01 = 0.2124 mol
Dividing through by the smallest mole
\(CoCl_2\) = 0.00257 / 0.00257 = 1
water = 0.2124/ 0.00257 = 83
Thus, the formula of the hydrate would be \(CoCl_2.83H_2O\)
More on hydrate salts can be found here: https://brainly.com/question/16990374
#SPJ1
How does the wind pattern during the day compare with the wind pattern at night?
Answers
20 points!!! PLEASE AWNSER ASAP!!!
A copper wire was cut into pieces and dropped into a test tube containing acid, Bubbles formed immediately, the liquid in the test tube turned green, and the pieces of wire were no longer visible, which most likely happened to the pieces of copper wire?
А. The copper wire was chemically changed when it was cut and placed in the acid,
B the copper wire was physically changed when it was cut and placed in the acid,
C The copper wire was physically changed when it was cut and chemically changed when it was placed in the acid,
D The copper wire was chemically changed when it was cut and physically changed when it was placed in the acid,
Answers
Answer: i think its A
Explanation:
Answer:
A
Explanation:
Becuase bubbles only come from chemical changes
Which statement is FALSE?
1) atoms in the same period have the same number of outer electrons
2) atoms in the same group behave in a similar manner during a chemical
reaction
3) the outer electrons are the electrons in the outer energy level of an atom
4) the number of outer electrons may be used to predict how an element would
react in a chemical reaction
Answers
Answer: I think the answer is 2.
Explanation:
The formula for calculating power is work divided by time (power = work + time). What are two ways of stating the same relationship?
work = power + time
nwork s power x time
power
time = work x power
Opower workx time
time
Work

Answers
Answer:
See the answer below
Explanation:
The formula for calculating power is such that;
power = work/time
Two ways to restate the relationship between the 3 variables would be to make time and work the subject of the formula respectively.
1. When time if made the subject of the formula;
time = work/time
2. When work is made the subject of the formula;
work = power x time
If a material is ductile, it is mostly likely a
nonmetal
metal
metalloid
Gas
Answers
Answer:
Explanation:
Which of the following is most likely to be ductile?
a. Metal
b. Nonmetal
c. Metalloid
d. Gas
Answer: a. MetalMetal