Which one of the following salts does not give a neutral solution when it is dissolved in water?
a. KNO3
b. BaCl2
c. Ca(ClO3)2
d. NH4OH
e. NaBr
Answers
The salt that does not give a neutral solution when dissolved in water among the options provided is d. NH4OH. A neutral solution has a pH of 7. When salts dissolve in water, they can form acidic, basic, or neutral solutions depending on the ions they release.
NH4OH is ammonium hydroxide, which dissociates into NH4+ (ammonium ion) and OH- (hydroxide ion) when dissolved in water. The presence of OH- ions increases the pH, making the solution basic rather than neutral. Therefore, among the given options, NH4OH does not form a neutral solution when dissolved in water. Most salts, like KNO3, BaCl2, Ca(ClO3)2, and NaBr, dissociate into a cation and an anion that do not affect the pH significantly, resulting in a neutral solution. However, NH4OH is different.
To know more about neutral solution
https://brainly.com/question/29510389
#SPJ11
Related Questions
.What is the KE of a baseball that has a mass of 100 kg and is traveling at 5 m/s?
Answers
Answer:
1250joules
Explanation:
The kinetic energy is energy in motion and it's formula is
KE =1/2MV^2
M represent mass which is 100Kg.
V represent velocity which is 5m/s
Therefore, KE = 1/2×100×5× 5
= 50 × 25
KE= 1250joules
A process which is unfavorable with respect to enthalpy, but favorable with respect to entropy Group of answer choices could occur at high temperatures, but not at lower temperatures. could not occur regardless of temperature. could occur at any temperature. could occur at low temperatures, but not at higher temperatures. none of the above
Answers
Answer:
could occur at any temperature.
Explanation:
The spontaneity of a reaction is what determines whether the reaction will occur or not. A spontaneous reaction occurs easily.
The spontaneity of a reaction is predicted by the sign of ∆G.
When ∆G is positive, the reaction is not spontaneous. When ∆G is negative, the reaction is spontaneous.
Note that;
∆G= ∆H - T∆S
Where;
∆H = Change in enthalpy
∆S = Change in entropy
T= temperature
If ∆H is unfavourable and ∆S is favourable, the reaction can proceed at all temperatures because ∆G will always be negative.
It should be noted that a process that is unfavorable as regards to enthalpy, but favorable with respect to entropy could occur at any temperature.
Enthalpy(∆H) serves as amount of internal energy that a compound has, entropy( ∆S) on the other hand serves as intrinsic disorder within the system.
However, spontaneity of a reaction determines likely hood of occurrence of a reaction, when the process is spontaneous, it makes the reaction to occurs easily.
We can conclude that whenever enthalpy is unfavourable and entropy of a system is favourable, then the spontaneity will be negative and the reaction will occur at any temperature.
Learn more about entropy and enthalpy on;
https://brainly.com/question/11753370
What is the specific name of the process for the reaction between the chromate ester and water?
Answers
The reaction between chromate ester and water is a part of a huge and consecutive reaction called Jones reaction.
In this reaction, alcohol is converted to chromic acid in presence of jones reagent, chromic acid is further converted to chromate ester.
Now the obtained chromate ester has one or more unstable alpha hydrogens and in presence of basic species like water it yields an aldehyde or ketone as the organic product.
O O
ll ll
R--R'CH--OH + H-O--Cr--OH ----> R-R'CH-O-Cr--OH +H₂O
ll ll
O O
Alcohol Chromic acid chromate ester
Further, let us understand the reaction of chromate ester with water with an example:
O O
ll ll
(CH₃)CHO---Cr---OH + H₂O ---->(CH₃)CO + Cr--OH +H₃O⁺
ll ll
O O
TO know more about ketone here
brainly.com/question/12308782
#SPJ4
4. Which of the following is true about used antifreeze?
OA. It can be treated just like new antifreeze
OB. It is not recycleable
OC. It can never be reused in a vehicle
OD. It often contains heavy metals
Answers
Answer:
D. It often contains heavy metals.
Explanation:
Used antifreeze should not be reused in a vehicle, it is not recyclable, and it should be disposed of properly because it often contains heavy metals, such as lead and copper, which can be harmful to the environment and human health. It should not be treated just like new antifreeze. It is important to check the antifreeze levels and quality regularly and replace it with fresh antifreeze as needed.
 Help ASAP only right answers only no spam don’t answer if you don’t know

Answers
i know the answer is a theory
β-dicarboxylic the hydrolysis of an alkylated malonic ester in aqueous sodium hydroxide followed by acidification with aqueous hydrochloric acid gives a(n)
Answers
β-dicarboxylic the hydrolysis of an alkylated malonic ester in aqueous sodium hydroxide followed by acidification with aqueous hydrochloric acid gives a carboxylic acid.
During the hydrolysis reaction, the ester bond of the alkylated malonic ester is cleaved, resulting in the formation of a β-dicarboxylic acid. This is due to the presence of two carboxylic acid groups (β-dicarboxylic) in the final product. When the alkylated malonic ester is treated with aqueous sodium hydroxide, the ester bond undergoes nucleophilic attack by hydroxide ions, leading to the formation of a β-keto acid intermediate. This intermediate then undergoes decarboxylation, resulting in the formation of a β-dicarboxylic acid.
Upon acidification with aqueous hydrochloric acid, the β-dicarboxylic acid is protonated, leading to the formation of its corresponding carboxylic acid. This is because the hydrochloric acid donates a proton to the β-dicarboxylic acid, neutralizing the negative charge on the carboxylate group. In summary, the hydrolysis of an alkylated malonic ester in aqueous sodium hydroxide followed by acidification with aqueous hydrochloric acid gives a carboxylic acid, specifically a β-dicarboxylic acid.
Learn more about hydrolysis reaction at:
https://brainly.com/question/16715756
#SPJ11
QUESTION 7 What is the pH of water? O pH12 O pH9 O pH7 O pH5 QUESTION 8 What is the pH when fish die from pollution? O pH12 O pH9 O pH7 O pH4 QUESTION 9 A solution with a pH less than 7 is basic. O True O False
Answers
7. The pH of water is pH7.
The pH scale measures the acidity or alkalinity of a substance. It ranges from 0 to 14, with pH7 considered neutral. Water has a pH of 7, indicating that it is neither acidic nor basic. It is important to note that the pH of pure water can vary slightly due to the presence of dissolved gases and minerals, but it generally remains close to pH7.
8. When fish die from pollution, the pH is typically around pH4.
Pollution can introduce harmful substances into water bodies, leading to a decrease in pH. Acidic pollutants, such as sulfur dioxide and nitrogen oxides, can cause the pH of water to drop significantly. When fish are exposed to highly acidic water, their physiological processes are disrupted, and they may die as a result. A pH of around pH4 is considered highly acidic and can be detrimental to aquatic life.
9. A solution with a pH less than 7 is acidic.
This statement is false. A solution with a pH less than 7 is actually considered acidic, not basic. The pH scale ranges from 0 to 14, with pH7 being neutral. Solutions with a pH below 7 are acidic, indicating a higher concentration of hydrogen ions (H+) in the solution. On the other hand, solutions with a pH above 7 are basic or alkaline, indicating a higher concentration of hydroxide ions (OH-) in the solution.
To know more about Pollutants visit-
brainly.com/question/29594757
#SPJ11
Elemental bromine reacts vigorously with elemental sodium metal to form a white solid. Does this characteristic of elemental bromine represent a physical or a chemical property?
Answers
The characteristic of elemental bromine reacting vigorously with elemental sodium metal to form a white solid represents a chemical property.
Chemical characteristics define how substances react or change chemically. A white solid forms when elemental bromine and sodium metal combine, suggesting a chemical transition.
However, a substance's physical attributes can be detected or quantified without changing its chemical composition. Colour, density, melting, and boiling points are physical qualities.
It is a chemical property of elemental bromine to react with sodium metal and generate a new compound.
Learn more about chemical property, here:
https://brainly.com/question/1728902
#SPJ4
2. What is true about the position of all metals in
the periodic table?
A. They are in the first group.
B. They are in the bottom period.
C. They are in the rightmost column.
D. They are to the left of the zigzag line.
Answers
Answer: D they are to the left of the zigzag line.
Explanation:
The first group are alkali metals and therefore wrong. The zigzag line is composed of semimetals and to the left we have metals like magnisum and metals are always bottom right in the table.
Ribosomes are cell structures that make
Answers
Ribosomes are cell structures that make protein. They are the primary site of the synthesis of protein. They are made up of protein and RNA.
What are ribosomes?Ribosomes are cell organelles scattered in the cytoplasm of the cell. They are tiny and round vesicles. They are present in both prokaryotic and eukaryotic cells. Furthermore, they are present freely in the cytoplasm. Ribosomes are also present near the endoplasmic reticulum.
Ribosomes are made up of RNA and protein, and they synthesize the protein in the cell. Ribosomes read the mRNA and by these codes of mRNA, it constitutes amino acids, that grow into a long chain of the protein.
Thus, ribosomes are the parts of cells that produce protein. They serve as the central location for protein production. They are composed of RNA and protein.
To learn more about ribosomes, refer to the link:
https://brainly.com/question/241631
#SPJ6
For each solute, identify the better solvent: water or carbon tetrachloride.
C6H6, I2, Na2S, CH3OH
Answers
The better solvent for each solute is as follows: (C\(_6\)H\(_6\) : carbon tetrachloride) (I\(_2\) : carbon tetrachloride) (Na\(_2\)S: water) (CH\(_3\)OH: water)
A solute is a substance that gets dissolved in a solvent to form a solution. A solvent is a substance that dissolves the solute. A solute is a substance that dissolves in a solvent to form a solution.
The solubility of a solute in a solvent can be affected by many factors, including the temperature, pressure, and chemical properties of the solute and the solvent. The solubility of a solute in a solvent can be measured using various techniques, such as titration, conductivity, and spectrophotometry.
In this case, the better solvent for each solute is determined as follows:
C\(_6\)H\(_6\) : carbon tetrachloride
The solubility of benzene (C\(_6\)H\(_6\)) is greater in nonpolar solvents such as carbon tetrachloride than in polar solvents such as water.
I\(_2\) : carbon tetrachloride
The solubility of iodine (I\(_2\)) is greater in nonpolar solvents such as carbon tetrachloride than in polar solvents such as water.
Na\(_2\)S: water
The solubility of sodium sulfide (Na\(_2\)S) is greater in polar solvents such as water than in nonpolar solvents such as carbon tetrachloride.
CH\(_3\)OH: water
The solubility of methanol (CH\(_3\)OH) is greater in polar solvents such as water than in nonpolar solvents such as carbon tetrachloride.
Learn more about the solution here:
https://brainly.com/question/1426795
#SPJ11
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
When a car driver puts their foot on the brake pedal, the brake fluid is pushed down a narrow tube connected to the brake. Since brake fluid is hard to squash, the brakes are put on immediately. What is the scientific term for squash?
Answers
When a car driver puts their foot on the brake pedal, the brake fluid is pushed down a narrow tube connected to the brake. Since brake fluid is hard to squash, the brakes are put on immediately the scientific term for squash is compressibility.
What is compressibility?The scientific term for squash is "compressibility". In the case of brake fluid, it has very low compressibility, which means that it cannot be easily compressed or squashed, resulting in immediate and effective brake application when pressure is applied to the brake pedal.
The term "compressibility" describes how much a particular volume of matter shrinks under pressure. A solid or a liquid under pressure essentially maintains its volume. The solid or liquid's constituent atoms, ions, or molecules are in close proximity to one another.
Learn more about driver at:
https://brainly.com/question/30774200
#SPJ1
[H+] for a solution is 1 x 10^-7 M. This solution is _____________.
A. acidic
B. basic
C. neutral
Answers
Answer:
the answer is C neutral
Explanation:
if the ph is lower then 7 its acidic
if its higher then 7 its basic
and a ph of 7 is neutral
The manufacturer of
a new convection oven claims that his product
will bake things 25 percent faster than a con-
ventional oven that relies mainly on radiation.
How could you test this claim?
Answers
According to studies, convection ovens generally cook 25% faster than traditional ovens at a lower cooking temperature of roughly 25 degrees F, even if cooking times vary slightly between ovens.
Convection ovens, in contrast to conventional radiant (also known as thermal) ovens, include a fan that evenly distributes air inside the chamber, removing hot and cold regions.
A convection oven may cook food up to 25% more quickly than a regular oven.
Food cooks roughly 25% more quickly in a convection oven because hot air is blown directly onto it rather than merely surrounding it.
Convection ovens heat up more quickly and cook food more quickly. Thanks to some straightforward modifications to the appliance, they also cook more evenly.
All of this results in tastier baked goods, meats, and other foods.
Overall, the convection oven option is excellent if you want a crisp, rapid result, but stick with the regular oven if you want your food to retain moisture or rise before it is fully baked.
To learn more about Convection oven from given link
https://brainly.com/question/15700369
#SPJ1
(science)
if you have visited a natural history museum, you have probably seen the dinosaur exhibits. of course, dinosaurs do not exist today. we know about them because of the discovery of what?
a. herds
b. fossils
c. stories
d. hatchlings
Answers
b. fossils we know about them because of the discovery .
What proof does science have that dinosaurs ever lived on Earth?The Earth was the home of the dinosaurs for at least 230 million years, according to a wealth of fossilized bones, teeth, trackways, and other physical evidence. But no dinosaur bones have been discovered in strata younger than 66 million years old, not even a single trace.
In June 2022, Dippy returned to the Natural History Museum as a component of a traveling exhibition. The replica will then be loaned to another institution for a period of three years. Applications for the future location were made available by the Museum in March 2022.
Learn more about fossils refer
https://brainly.com/question/19083813
#SPJ4
Plssss helppp quick it’s for science and I don’t get it

Answers
Question 5 OT 5
At which temperature do particles stop moving entirely?
O A. 0°C
O B. 32 K.
O C. 32°F
O D.OK
Answers
physical and chemical proportys
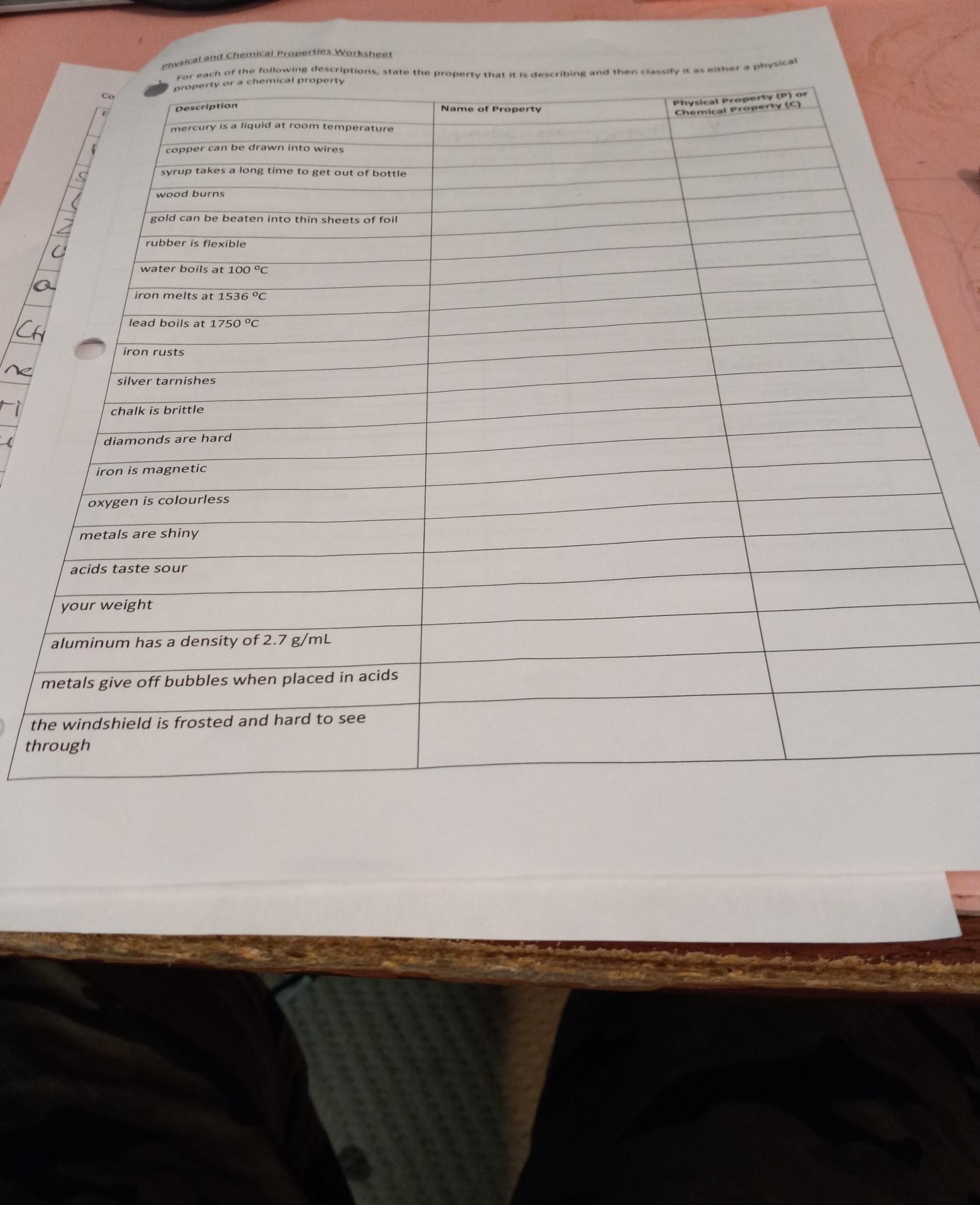
Answers
The physical and the chemical properties of matter are listed below.
What are the examples of physical and chemical properties?While the entire page is not clear, I have an idea of what you are trying to ask and I would show you the physical and the chemical properties of matter.
Physical properties are characteristics of a substance that can be observed or measured without changing the substance's chemical composition. Examples of physical properties include:
Density
Color
Boiling point
Melting point
Hardness
Solubility
Electrical conductivity
Optical properties (such as refractive index)
Chemical properties are characteristics of a substance that describe its ability to undergo chemical reactions and form new substances. Examples of chemical properties include:
Reactivity with other substances
Combustibility
Oxidation state
Acidity or basicity (pH)
Toxicity
Flammability
Learn more about matter:https://brainly.com/question/28487167
#SPJ1
when aluminum (al) reacts with oxygen (o2) in a synthesis reaction, what is the chemical formula of the product? group of answer choices alo2 none of these options al2o3 al3o2
Answers
This is a synthesis reaction we're given that aluminium (Al) can react with oxygen gas (O2 to produce aluminium oxide (Al2O3).The balanced chemical equation for this reaction is 4Al(s)+3O2(g)→2Al2O3(s)
When two separate atoms or molecules interact to create a new compound or molecule, the process is known as a "synthesis reaction." The majority of the time, energy is released and an exothermic reaction takes place during a synthesis reaction. An endothermic result is also conceivable, though. When sodium (Na) and chlorine (Cl) are combined, sodium chloride is created. This is an illustration of a synthesis reaction (NaCl). Al2O3 is the chemical formula for the compound of aluminium and oxygen known as aluminium oxide. It is precisely referred to as aluminum(III) oxide and is the most prevalent of various aluminum oxides. Aluminum oxide is utilized for its strength and hardness.
Learn more about synthesis reaction here:
https://brainly.com/question/16987748
#SPJ4
2 3 4 0
1 H -> 1 H- -> 3He + n is an example of what type of nuclear reaction (1.)
235 0 92 141 0
(2.) U + n -> 35 Kr + 56 Ba + 3n is the example fission or fusion? explain.
please label the answers to which one they go to. for number one 2 is over one 3 is over 1 and 4 is over Two by H and He and 0 is over N. for number two 235 is by U 0 is by n 92 goes over 35 by Kr and 141 is over 56 by Ba and 0 is by 3n.
Answers
Reaction 1 is nuclear fusion
Reaction 2 is nuclear fission
What is nuclear fission and nuclear fusion?Nuclear fission is the process in which the nucleus of an atom is split into two or more smaller nuclei while Nuclear fusion, on the other hand, is the process in which two or more atomic nuclei combine to form a larger nucleus.
In reaction 1, there is the combination of hydrogen nuclei while in reaction 2 we have the breaking apart of a uranium nuclei. This is fission and fusion reactions respectively.
Learn more about nuclear reaction:https://brainly.com/question/10687690
#SPJ1
An element found in groups 3-12 of the periodic table is classified as a[n]
Answers
Answer:
Transition Metals
Explanation:
Hope this helped :)
Which reaction is exothermic?
Answers
Answer:
Exothermic reactions are reactions in which energy is released in the form of light, heat, etc.
for example :-
=》CaO + H2O -> Ca(OH)2
Look at the Recording station detector on the upper left side of the Gizmo. What happens when the seismic waves hit the recording station?
Answers
Answer:
I don’t know what recording station you’re referring to but, When seismic waves reach the seismograph, a graphical record, or seismogram, is produced
Explanation:
The seismic waves hitting the recording station has been resulted in the seismograph, that has been evident of the earthquake.
The seismic wave has been the radiation, with the result of the movement of the earth surface. The movement has been result in the earthquake.
The intensity of the earthquake has been measured by seismograph on the Richter scale. The seismic wave results in the movement of the leads to the production of the seismograph.
The seismic waves hitting the recording station has been resulted in the seismograph, that has been evident of the earthquake.
For more information about earthquake, refer to the link:
https://brainly.com/question/1296104
consider the reaction of alcohol dehydrogenase. ethanol nad acetaldehyde nadh h which is the reducing agent for this reaction?
Answers
In the hydrogenation of alcohol, Ethanol is the reducing agent and it loses electron.
Alcohol hydrogenase transform ethanol to Acetaldehyde. This known as carcinogen. In this Acetaldehyde is more toxic compound. Alcohol dehydrogenases catalyze the oxidation of primary and secondary alcohols to the corresponding aldehyde or ketone by the transfer of a hydride anion to NAD+ with release of a proton.
Ethanol + NAD^+ ---> acetaldehyde + NADH + H^+
The half reactions are:
Ethanol --------->Acetaldehyde + 2H^+ + 2e (oxidation)
NAD+ + 2H+ + 2e- ------->NADH + H^+ (Reduction)
Ethanol is oxidized to acetaldehyde, because it loses e- .NAD+ is reduced to NADH, because it gain of e− . NAD is the oxidizing agent. Oxidizing agent gains e− and therefore reduced during reaction. Ethanol is the reducing agent. Reducing agent loses e−and therefore oxidized during reaction . Alcohol dehydrogenase is also involved in the toxicity of other types of alcohol.
To know more about Dehydrogenation please visit:
https://brainly.com/question/16026648
#SPJ4
what volume of water is needed to dissolve 2.70 grams of n2 at 25 oc under a pressure of 4.46 atm? kh for n2
Answers
2.70 grammes of N₂ must dissolve in 30.5 litres of water at 25 degrees Celsius and 4.46 atm of pressure.
The solubility of N₂ in water depends on the temperature and pressure. To determine the volume of water needed to dissolve 2.70 grams of N₂ at 25 °C and 4.46 atm, we need to use the Henry's law equation, which relates the solubility of a gas in a liquid to its partial pressure:
C = kH x P
where C is the concentration of the gas in the liquid, kH is the Henry's law constant for the gas, and P is the partial pressure of the gas above the liquid.
The Henry's law constant for N₂ in water at 25 °C is 7.07 x 10⁻⁴ M/atm.
First, we need to convert the mass of N₂ to moles using its molar mass:
moles of N₂ = 2.70 g / 28.02 g/mol = 0.0963 moles
Next, we can use Henry's law equation to find the concentration of N₂ in water:
C = kH x P = (7.07 x 10⁻⁴ M/atm) x (4.46 atm) = 3.16 x 10⁻³ M
Finally, we can use the definition of concentration (C = moles of solute / volume of solvent) to solve for the volume of water needed:
Volume of water = moles of solute / concentration = 0.0963 moles / 3.16 x 10⁻³ M = 30.5 L
Therefore, 30.5 liters of water are needed to dissolve 2.70 grams of N₂ at 25 °C under a pressure of 4.46 atm.
To learn more about volume refer to:
brainly.com/question/14710169
#SPJ4
Trace amounts of oxygen gas can be "scrubbed" from gases using the following reaction: 4 Cr2+(aq) + O2(g) + 4 H+(aq)-4 Cr3+(aq) + 2 H2O(l) Which of the following statements is true regarding this reaction? A. O2 (g) is reduced B. Cr2+(aq) is the oxidizing agent. C. O2(g) is the reducing agent. D. Electrons are transferred from 02 to Cr2-
Answers
In the reaction 4 Cr²⁺(aq) + O₂(g) + 4 H⁺(aq) → 4 Cr³⁺(aq) + 2 H₂O(l), trace amounts of oxygen gas are removed from the mixture. This reaction involves redox processes, where oxidation and reduction occur simultaneously. The correct options are A and B.
A. O₂ (g) is reduced: This statement is true. In the reaction, the oxygen gas (O₂) gains electrons, changing its oxidation state from 0 to -2 (in H₂O). Gaining electrons is the process of reduction.
B. Cr²⁺(aq) is the oxidizing agent: This statement is also true. The oxidizing agent is the substance that causes the reduction of another species. In this case, Cr²⁺ causes the reduction of O₂ by accepting electrons and undergoing a change in its oxidation state from +2 to +3.
C. O₂(g) is the reducing agent: This statement is false. The reducing agent is the substance that causes the oxidation of another species. In this reaction, O₂ is reduced, not the reducing agent. The reducing agent is Cr²⁺, as it loses electrons and causes the oxidation of other species.
D. Electrons are transferred from O₂ to Cr²⁺: This statement is false. Electrons are transferred from Cr²⁺ to O₂. Cr²⁺ loses electrons and gets oxidized to Cr³⁺, while O₂ gains electrons and gets reduced to form H₂O.
To know more about redox processes, refer to the link below:
https://brainly.com/question/31959470#
#SPJ11
Metal or Non-metal?
Potassium: metal
Fluorine:
Bromine:
Hydrogen
Beryllium:
Nitrogen
Answers
Answer:
Fluorine: non-metal
Bromine: non-metal
Hydrogen: non-metal
Beryllium: metal
Nitrogen:non-metal
Is Los Angeles affected by ocean acidification?
Answers
Answer:
No son los angeles pero en realidad....
Explanation:
¿Qué provoca la acidificación de los océanos?
Cuando el CO2 es absorbido por el océano, se producen reacciones químicas. En particular, se forma ácido carbónico y se liberan iones de hidrógeno; como resultado, el pH de las aguas superficiales del océano disminuye, haciéndolas más ácidas.
Why is the standard entropy of a substance in the gas state greater than its standard entropy in the liquid state?
Answers
The standard entropy of a substance in the gas state is generally greater than its standard entropy in the liquid state due to the greater molecular disorder and freedom of motion of the gas molecules compared to those in the liquid state.
In the gas state, the molecules have much more kinetic energy and are able to move freely and independently from each other, allowing them to occupy a larger volume and explore a greater number of possible states. This means that there are many more ways for the gas molecules to be arranged than in the liquid state, resulting in a greater degree of randomness or disorder. In contrast, in the liquid state, the molecules are more closely packed together and have less freedom of motion due to intermolecular forces of attraction. The number of possible states of the liquid molecules is therefore more limited than that of the gas molecules, resulting in a lower degree of randomness or disorder. Since entropy is a measure of the degree of randomness or disorder in a system, the greater molecular disorder and freedom of motion in the gas state leads to a greater standard entropy compared to the liquid state for the same substance.
Learn more about standard entropy here:
https://brainly.com/question/30691597
#SPJ11