Which pair of atoms have similar properties?
a. mg and br
b. li and be
c. cl and br
d. f and he
e. li and f
Answers
Cl and Br have similar properties as they belong to halogen family.
What is halogen family?Halogen, any of the six nonmetallic elements that constitute Group 17 (Group VII A) of the periodic table. The halogen elements are fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and (Ts). They were given the name halogen, from the Greek roots Hal- (“salt”) and -gen (“to produce”), because they all produce sodium salts of similar properties, of which sodium chloride—table salt, or halite—is best known.
Because of their great reactivity, the free halogen elements are not found in nature. In combined form, fluorine is the most abundant of the halogens in Earth’s crust. The percentages of the halogens in the igneous rocks of Earth’s crust are 0.06 fluorine, 0.031 chlorine, 0.00016 bromine, and 0.00003 iodine. At and Ts do not occur in nature, because they consist of only short-lived radioactive isotopes.
Chlorine is the best known of the halogen elements. The free element is widely used as a water-purification agent, and it is employed in a number of chemical processes. In the past ethylene dibromide was extensively used as an additive in.
To know more about Chlorine visit: https://brainly.com/question/14962130
#SPJ4
Related Questions
halogens are found combined together in molecules which contain ........ atoms joined together.
a) 2
b) 3
c) 5
d) 4
Answers
how many unpaired electrons are in the cobalt atom?
Answers
Cobalt atom (Co) has one (1) unpaired electron in ground state.
How many unpaired electrons are in the cobalt atom?The cobalt atom (Co) has multiple oxidation states, which means it can have different numbers of unpaired electrons depending on its charge.
In its neutral state (Co⁰), cobalt has a total of 27 electrons.
If we split the 27 electrons as follows; we will have;
2, 8, 8, 8, 1
From the electron configuration given above, we can see that the outer most electron of cobalt is 1, meaning that is has only 1 unpaired electron in ground state.
Thus, cobalt atom (Co) has one (1) unpaired electron in ground state.
Learn more about unpaired electron here: https://brainly.com/question/12368969
#SPJ4
1. How many moles of potassium are present in 4.23 x 1025 potassium atoms?
(show solution)
please help if u know only. ty
Answers
Answer:
70.26 moles
Explanation:
1 mole of potassium will contain 6.02 x 10²³ atoms of potassium
6.02 x 10²³ atoms of potassium = 1 mole
4.23 x 10²⁵ atoms of potassium = 4.23 x 10²⁵ / 6.02 x 10²³
= 70.26 moles .
Molal boiling-point-elevation constant (Kb ) for pure water is 0.51 0C/ m. A dilute solution of a nonvolatile solute (does not dissociate) in water was found to boil at 105.1 0C. The concentration of the nonvolatile solute in the solution is ----------------- m.
Answers
Molal boiling-point-elevation constant (Kb ) for pure water is 0.51 0C/ m. A dilute solution of a nonvolatile solute (does not dissociate) in water was found to boil at 105.1 0C. The concentration of the nonvolatile solute in the solution is 1.18 m.
A molal boiling-point-elevation constant is a measure of the elevation of a solvent's boiling point. This is achieved by dissolving a solute in a solvent. The solvent is given a higher boiling point by the solute. Molality, which is a measure of the number of moles of solute in a solvent per kilogram of solvent, is used to calculate the boiling point elevation. It is a temperature change resulting from the addition of solute to the solvent. Molal boiling-point-elevation constant (Kb ) for pure water is 0.51 0C/ m. A dilute solution of a nonvolatile solute (does not dissociate) in water was found to boil at 105.1 0C. To calculate the molarity of a solution, the following formula is used:
ΔTb = Kb x m x i.
ΔTb is the boiling point elevation, Kb is the molal boiling-point-elevation constant of the solvent, m is the molality of the solute in the solution, and i is the van't Hoff factor.
The boiling point elevation is calculated by subtracting the boiling point of the solvent from the boiling point of the solution. The boiling point of pure water is 100°C.
ΔTb = 105.1 - 100 = 5.1.
We know that the molal boiling-point-elevation constant (Kb ) for pure water is
0.51 0C/ m.
So, 5.1 = 0.51 x m.
The value of m can be calculated as follows:
m = 5.1 / 0.51 = 10.
Therefore, the concentration of the nonvolatile solute in the solution is 1.18 m.
Learn more about boiling-point-elevation visit:
brainly.com/question/30641033
#SPJ11
a solution contains one or more of the following ions: ag ag , ca2 ca2 , and cu2 cu2 . when you add sodium chloride to the solution, no precipitate forms. when you add sodium sulfate to the solution, a white precipitate forms. you filter off the precipitate and add sodium carbonate to the remaining solution, producing another precipitate.
Answers
SO₄⁻² (aq) + Ca⁺₂(aq) → CaSO₄(s)
CO₃⁻²(aq) + Cu⁺²(aq) → CuCO₃(s).
when you add sodium chloride to the solution, no precipitate forms. when you add sodium sulfate to the solution, a white precipitate forms. you filter off the precipitate and add sodium carbonate to the remaining solution, producing another precipitate.
When sodium chloride (NaCl) is added to the solution, sodium chloride dissociates and forms the ions Na+ and Cl-. The anion can be form salts with the other cations presented in the solution. According to the solubility table of the salts, AgCl is a nonsoluble salt, and so, it was expected to form a precipitate. Perhaps, the concentration was not higher enough to do this.
When sodium sulfate (Na2SO4) is added, sodium sulfate dissociates and forms Na+ and SO₄⁻², the sulfate ion can react with the other cations. According to the solubility table, the salt CaSO4 (Ca is from group 2) is nonsoluble, so it will be formed. The net ionic equation represents the ions in solution that reacts:
Na⁺(aq) + SO₄⁻² (aq) + Ca⁺₂(aq) → Na⁺(aq) + CaSO₄(s)
SO₄⁻² (aq) + Ca⁺₂(aq) → CaSO₄(s)
When sodium carbonate (Na2CO3) is added, it dissociates and forms Na+ and CO₃⁻². According to the solubility table, the salt CuCO₃ is nonsoluble and will form a precipitate:
Na⁺(aq) + CO₃⁻²(aq) + Cu⁺²(aq) → Na⁺(aq) + CuCO₃(s)
CO₃⁻²(aq) + Cu⁺²(aq) → CuCO₃(s)
Learn more about sodium sulfate here:
https://brainly.com/question/4025301
#SPJ4
A solid produced by a chemical reaction in solution that separates from the solution is called?
Answers
A solid produced by a chemical reaction in solution that separates from the solution is called Precipitate.
Precipitate: a solid that forms from a chemical reaction taking place in solution and separates from the solution.
It can also be formed by passing a gas into an aqueous solution of a substance (like passing carbon dioxide into lime water).a solid created when a solution undergoes a change, frequently as a result of a chemical reaction or temperature shift that makes a solid less soluble. A precipitate in meteorology is either liquid or solid water (rain, snow, etc.)
The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the 'Supernate' or 'Supernatant'.
Numerous instances of mineral production in nature can be attributed to precipitation reactions, such as metal sulphide creation at so-called "black smokers," submarine vents.Therefore, the chemical reaction in solution that separates from the solution is called precipitate.
For more such questions on Precipitate.
https://brainly.com/question/18109776
#SPJ4
Please help with these two (last question is nwse)

Answers
Answer:
your answer gonna be 20 miles
5 of 255 of 25 Items
06:09
Question
Predict which compound would have the highest melting point and explain why.
Responses
A SiO2 - It consists of a covalent network.SiO 2 - It consists of a covalent network.
B CO - It is polar and has dipole-dipole forces.CO - It is polar and has dipole-dipole forces.
C P2O5 - It has strong dipole-dipole forces.P 2 O 5 - It has strong dipole-dipole forces.
D CO2 - It has really strong dispersion forces.
Answers
The melting point of silicon dioxide SiO₂ is higher than the other given compounds because, It consists of a strong covalent network and the bond between Si-O is strong.
What is melting point ?Melting point of a substance is the temperature at which it converts from its solid state to liquid state where the solid phase and liquid phase are in equilibrium.
Melting point of the substance depends on bond type, molar mass, temperature, pressure and presence of impurities. It increases with increase in the strength of intermolecular bonds.
The more the bond strength more energy needed to apply to weaken the bonds and melt the compound. Among the given compounds, silicon dioxide is having stronger covalent bonds. Hence, option A is correct.
Find more on melting point:
https://brainly.com/question/29578567
#SPJ1
If you have 1 mol xe and 1 mol f₂, how many moles of xef₄ can you create in the following chemical reaction? xe (g) 2 f₂ (g) → xef₄ (g).
Answers
The number of moles of XeF4 that can be created would be 0.5 moles.
From the equation of the reactiobn:
Xe (g) 2 F₂ (g) ---> XeF₄ (g)
The mole ratio of Xe to F2 is 1:2. Hence, in the presence of 1 mole Xe and 1 mole F2, F2 would be limiting the reaction.
This means that the amount of F2 would determine the amount of XeF4 that would be produced from the reaction.
The mole ratio of F2 to XeF4 according to the equation is 2:1. This means that for every 1 mole of F2 introduced into the reaction, 0.5 moles of XeF4 will be produced.
In this case, 1 mole of F2 is introduced. Thus, the mole of XeF4 that would be produced would be:
1/2 = 0.5 moles.
More on stoichiometric calculations can be found here: https://brainly.com/question/8062886?referrer=searchResults
Please help me if you can. (I will give branliest to whoever answers first and if they have a reasonable answer.)

Answers
Tell : [Kr] 5s2 4d10 5p4
Radium : [Rn] 7s2
Lawren: [Rn] 5f14 7s2 7p1
Hope this helps!!!
calculate the volume occupied by 2.2 gram of Co2 at 27 degree Celsius and 1 bar pressure class 11
Answers
Answer:
1.255 L
Explanation:
Assuming that carbon dioxide acts as an ideal gas given the following conditions, using the ideal gas equation shown below
\(pV \ = \ nRT\),
and since
\(n \ = \ \displaystyle\frac{m}{M_{r}}\),
rewriting and rearranging the prior equation to make the variable \(V\) the subject, yields
\(V \ = \ \displaystyle\frac{mRT}{pM_{r}}\),
where \(V\) is the volume occupied by the gas, \(m\) is the mass of the gas, \(R\) is the gas constant, \(T\) is the temperature in Kelvins, \(p\) is the pressure exerted by the gas and \(M_{r}\) is the molecular weight of the gas molecule.
Therefore, plugging the given values into the rearranged equation,
\(V \ = \ \displaystyle\frac{(2.2 \ \text{g})(0.08314 \ \text{L} \ \text{bar} \ \text{mol}^{-1} \ \text{K}^{-1})(27 \ + \ 275) \text{K}}{(1 \ \text{bar})(12 + 2 \times16)\text{g mol}^{-1}} \\ \\ V \ = \ 1.255 \ \text{L} \quad \text{(4 s.f.)}\)
The following equation is balanced according to the Law of Conservation of Mass:
2 NO + O2 → 2 NO2
True
False
Answers
True
the side chain of which amino acid is most likely to form a hydrogen bond with the side chain of glutamate?
Answers
The side chain of which amino acid is most likely to form a hydrogen bond with the side chain of glutamate is: lysine.
The side chain of lysine is most likely to form a hydrogen bond with the side chain of glutamate. Lysine and glutamate are both amino acids. Amino acids are the building blocks of proteins. The two side chains of amino acids can interact via hydrogen bonding.
A hydrogen bond is a non-covalent interaction between two electronegative atoms in which hydrogen is bonded to one atom and electronegative (N, O, or F) is bonded to another atom. These types of interactions are crucial in the stabilization of proteins and nucleic acids.
Amino acids that interact via hydrogen bonding play a key role in stabilizing the three-dimensional structure of proteins. Proteins can be stabilized by hydrogen bonding in a variety of ways, including within the polypeptide backbone, between the polypeptide backbone and side chains, and between two side chains.
To know more about "Amino acid" refer here:
https://brainly.com/question/16734198#
#SPJ11
When 8.0 g H₂ react with 8.0 g O₂ in the reaction 2H₂ + O₂ → 2H₂O, what are the theoretical yield and the limiting reactant?
Answers
Answer:
Now, we have to determine the limiting reagent.
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g of
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.Now, 4 g of H₂ forms 36 g of H₂O
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.Now, 4 g of H₂ forms 36 g of H₂O1 g of H₂ forms 36/4 g of H₂O. 3 g of H₂ forms 36/4 x 3 = 27 g of H₂O
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.Now, 4 g of H₂ forms 36 g of H₂O1 g of H₂ forms 36/4 g of H₂O. 3 g of H₂ forms 36/4 x 3 = 27 g of H₂OMaximum amount of water that can be formed is 27 g.
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.Now, 4 g of H₂ forms 36 g of H₂O1 g of H₂ forms 36/4 g of H₂O. 3 g of H₂ forms 36/4 x 3 = 27 g of H₂OMaximum amount of water that can be formed is 27 g.For, amount of oxygen left of unreacted, Only 24 g of oxygen will react.
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.Now, 4 g of H₂ forms 36 g of H₂O1 g of H₂ forms 36/4 g of H₂O. 3 g of H₂ forms 36/4 x 3 = 27 g of H₂OMaximum amount of water that can be formed is 27 g.For, amount of oxygen left of unreacted, Only 24 g of oxygen will react.But 29 g is the given amount. Amount of oxygen unreacted = 29 - 24 = 5 g
The theoretical yield of the given chemical equation is 64 g and limiting reactant is oxygen.
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
As per the equation 4 g hydrogen reacts with 32 g oxygen thus 8 g hydrogen will react with 8×32/4=64 g oxygen.
Thus, the theoretical yield of the given chemical equation is 64 g .
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ7
What information does a radioactive element's half-life tell you about that element?
Answers
A liquid has a density of 0.70g/ml. Find the mass of the liquid which can be put
into a beaker holding 130mL.
Answers
Answer:
The answer is
91 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
density of liquid = 0.70 g/mL
volume = 130 mL
The mass is
mass = 0.7 × 130
We have the final answer as
91 gHope this helps you
2. would the separation of the two compounds in this experiment have been successful if the eluting solvent order had been reversed (1:2 hexane:ether first, hexanes second)? explain.
Answers
No, the separation of two compounds would not have been successful, if 1:2 (hexane:ether) was used first both compounds would move down the column together.
Separating mixtures into Their pure components are an important part of organic chemistry. For example, a chemist wants to purify a crude extract of a medicinal plant.
Column Chromatography : This technique is performed by packing the adsorbent ina glass tube, as shown below. There are many types of adsorbents (solid and phases)used in column chromatography, and the choice of adsorbent depends on the typeof compounds to be separated. Used Adsorbent in it are silica gel and alumina. Silica gel is used to separate various compounds such as hydrocarbons, alcohols, ketones, esters, acids, and azo compounds and amines. The column can be developed with a single solvent or a solvent gradient. For example, if the column is initiallydeveloped with a less polarsolvents such as hexane and evolution evolvesas fractions are collected, changethe solvent to 1:2 hexane-methylene chloride. Polar gradients areused for mixtures of compounds with widely different polarities.
Solvent : A common non-polar solvent in both thin layer and chromatography is hexane. Can be used for various polar solvents.
Elution Sequence: The approximate elution sequence is primarily based on polarity and compounds arehydrocarbons, olefins, ethers, halocarbons, aromatics, ketones, aldehydes, esters, alcohols, amines, and acids.
Important point is that the more polar the solvent, the faster The compound will elute, regardless of the compound's polarity.
To learn more about Chromatography, refer:
https://brainly.com/question/17143033
#SPJ4
PLEASE HELP HELP ME. THIS IS DUE TODAY PLEASE

Answers
Answer:
C
Explanation:
cause i'm smart
Answer:
D
Explanation:
Because it says tree roots breaking rocks into smaller rocks.
Hope this helped!
hydrogen, when combined with oxygen in a chemical reaction, forms water. this is an example of which type of chemical reaction?
Answers
Hydrogen, when combined with oxygen in a chemical reaction, forms water. this is an example of Combination reaction.
A chemical reaction is a process in which one or more substances, which are also called reactants, are generally converted to one or more different substances, that are known as the products. Substances formed are either chemical elements or compounds.
A chemical reaction usually rearranges the constituent atoms of the reactants to create different substances as products. The properties of the products are generally different from those of the reactants.
Formation of water from hydrogen and oxygen is a combination reaction because hydrogen and oxygen are combining to form one single product.
A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called as the synthesis reactions. The general form of a combination reaction is: A+B→AB. One combination reaction is two elements combining to form a compound.
To know more about combination reaction
https://brainly.com/question/3664113
#SPJ4
Which is TRUE about intermolecular
forces?
They are STRONGER than a chemical
bond
They are WEAKER than a chemical bond
Answers
Answer:
They are Weaker than a chemical
bondcorrected by the one in the comment section
15g of hydrogen gas reacts exactly with 70g of nitrogen gas to produce ammonia, NH3. deduce the balanced equation for the reaction.
Answers
Answer:
3H2 + N2 ---> 2NH3
Explanation:
please put the following statements in the correct order relating to the day of the lab practical. number 1-6: come to the help session the week of the lab practical. do at least 3 good trials (weigh and titrate sample) as time allows. start the lab practical by diluting the naoh solution. calculate the molarity of the dilute naoh, the percent by mass of the compound in you unknown for each trial and then the median. wash all your glassware and unknown vials, checkin your locker, evaluate the lab, and check your grades. the week before the practical clean glassware, read the practical instructions, do the practice problem in the lab manual, and complete the online problems for the final.
Answers
To prepare for the lab practical, it is important to clean glassware and read the practical instructions, as well as complete practice problems in the lab manual and online problems for the final the week before.
During the lab practical, the first step is to dilute the NaOH solution, followed by conducting at least three good trials and calculating the molarity of the dilute NaOH and the percent by mass of the compound in your unknown for each trial and then finding the median. After completing the experiments, it is important to wash all glassware and unknown vials, check your locker, evaluate the lab, and check your grades. Finally, it is recommended to attend the help session the week of the lab practical to clarify any doubts and ensure a successful practical experience.
To learn more about glassware refer:
https://brainly.com/question/1906681
#SPJ11
I need help getting this done
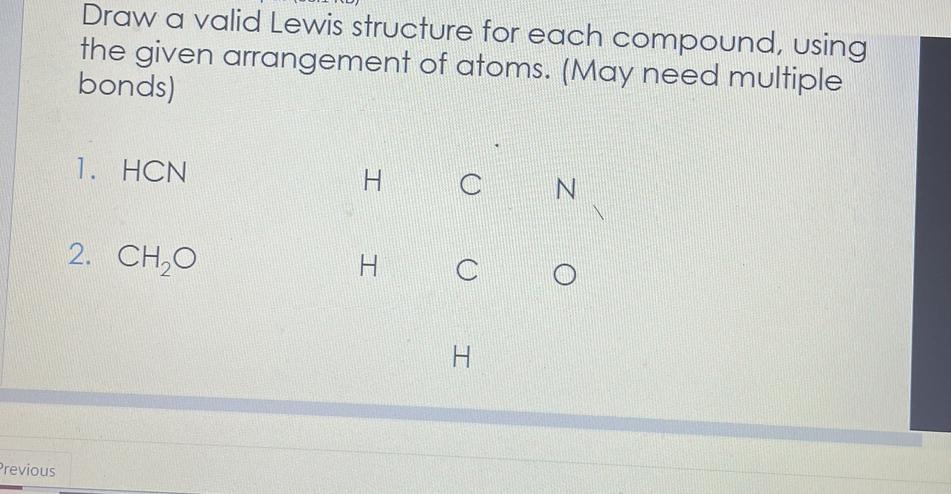
Answers
Answer:
I put the right structures in the pictures for you


a 1) How would you make 1 liter of a 10% NaCl solution from a solid stock? Provide details of what kind of containers you would use.
Answers
To make 1 liter of a 10% NaCl solution from a solid stock, you will require the following materials and containers.MaterialsSolid NaClDistilled water1-Liter volumetric flask250-mL volumetric flask 2-beakersProcedureTo prepare 1 liter of a 10% NaCl solution, the following procedure should be followed:Measure out 100g of NaCl using a balance.
Measure the weight of an empty 250-mL volumetric flask.Add the NaCl to a 250-mL beaker and add a small amount of distilled water to it to dissolve the NaCl.Carefully pour the dissolved NaCl solution into the 250-mL volumetric flask. Add distilled water to the mark on the flask to make up the volume. Stopper the flask and invert it several times to mix the solution.Measure the weight of the 1-Liter volumetric flask.Add the 250-mL volumetric flask solution to a 1-Liter volumetric flask.Add distilled water to the mark on the flask to make up the volume.
Stopper the flask and invert it several times to mix the solution.The final volume of the solution will be 1 liter of a 10% NaCl solution.PrecautionsEnsure the NaCl has completely dissolved before adding more water to avoid making a less concentrated solution.Measure the weight of the volumetric flask before and after adding the solution to calculate the volume of solution that was added.Use distilled water to prepare the solution.
To know more about volumetric flask visit:-
https://brainly.com/question/28997155
#SPJ11
why does ph change more with weak acids
Answers
Weak acids dissociate partially in the water, releasing fewer protons (H+) than strong acids. This results in a lower pH compared to strong acids because there are fewer protons available to form hydrogen ions.
The pH changes more with weak acids because they do not completely dissociate in water. This means that there are fewer hydrogen ions available to affect the pH. Strong acids, on the other hand, completely dissociate in water, meaning that they release all of their hydrogen ions and have a greater effect on the pH.
For example, let's compare hydrochloric acid (HCl), a strong acid, and acetic acid (CH₃COOH), a weak acid. When HCl is added to water, it completely dissociates into H+ and Cl- ions, resulting in a large increase in the concentration of H+ ions and a large decrease in pH. However, when acetic acid is added to water, only a small fraction of the molecules dissociate into H+ and CH₃COO- ions, resulting in a smaller increase in the concentration of H+ ions and a smaller decrease in pH.
Learn more about weak acids at https://brainly.com/question/24018697
#SPJ11
Write equations that show the processes that describe the first, second, and third ionization energies for a gaseous aluminum atom.
Answers
The first, second, and third ionization energy are, respectively:
\(Al ---- > Al^{+} + 1e^{-} \\Al^{+} ---- > Al^{+2} + 1e^{-} \\Al^{+2}--- > Al^{+3} +1e^{-}\)
Describe the first, second, and third ionization energies for gaseous aluminum atoms.Electrons are held in atoms by their attraction to the nucleus, which means that energy is needed to remove an electron from the atom.
Ionization energy, also called ionization potential, is the necessary energy that must be supplied to a neutral, gaseous, ground-state atom to remove an electron from an atom. When an electron is removed from a neutral atom, a cation with a charge equal to +1 is formed.
So, in this case, the first ionization energy is the energy required to remove the valence electron(outermost) from a neutral atom and is expressed as:
\(Al ---- > Al^{+} + 1e^{-}\)
The second ionization energy represents the energy required to start a second electron. Its value is always greater than the first ionization energy because the volume of a positive ion is less than that of the neutral atom and the electrostatic force is greater in the positive ion than in the atom.
Then, in this case, the second ionization energy is expressed as:
\(Al^{+} ---- > Al^{+2} + 1e^{-}\)
Finally, the third ionization energy represents the energy necessary to start an electron from a positive ion and its value is always greater than that of the second ionization energy. This is because the volume of the ion is smaller, and because of this, the electrostatic force is higher.
So, in this case, the third ionization energy is expressed as:
\(Al^{+2}--- > Al^{+3} +1e^{-}\)
In summary, the first, second, and third ionization energy are, respectively:
\(Al ---- > Al^{+} + 1e^{-} \\Al^{+} ---- > Al^{+2} + 1e^{-} \\Al^{+2}--- > Al^{+3} +1e^{-}\)
Learn more about Ionization energy here:-
https://brainly.com/question/8755621
#SPJ4
Convert .0011 to scientific notation
Answers
The sentences below are things people might say if they were planning to invest or not planning to invest Sort them into the correct categories . am thinking about buying stocks Planning to Invest Not Planning to Invest don't know much about investing can't afford to buy stocks don't really like to take risks need a way to manage my money want to save money for my future
Answers
Investing :
"I'm considering investing in equities."
"I need a system for handling my finances."
"I need to put money away for the future."
No Investment :
"My knowledge of investment is limited."
"I am unable to purchase stocks."
"I don't particularly enjoy taking chances."
Explanation:
These are accurate because I recently completed the course that taught me these things.
Learn ore about investing and no investment from here :
https://brainly.com/question/4459036?referrer=searchResults
#SPJ10
What is the visual indicator that enough of a drying agent, such as anhydrous m g s o 4 or c a c l 2 , has been added to properly dry an organic solution?
Answers
The visual indicator that enough of a drying agent , such as anhydrous MgSO4 or CaCl2 has been added to properly dry an organic solution is that the drying agent will move freely like a powder around the solution .
Because the anhydrous form is hygroscopic ( readily absorbs water from the air ).
What is the work of anhydrous magnesium sulphate ?
Magnesium sulphate is frequently used in the laboratory as an indicator, especially after aqueous work-up . aqueous work-up is a common technique in the lab to get rid of residual impurities after completion of a reaction . for this the organic reaction is cooled to room temperature and mixed with water .specific impurities will then diffuse into the aqueous phase and can be separated with a separatory funnel .
unfortunately some residual water will stay in the organic phase and this can have negative impact for characterization of compound . therefore Magnesium sulphate is added ,which is able to catch the residual water in its crystal lattice .after filtration of the solid magnesium sulfate ,the water is reduced to non-significant amount and the compound ready for further investigation.
Similarly , calcium chloride is strongly hygroscopic ( absorbs water from the environment ) , so it removes moisture from the air ,making it dryer . this results in water in the substance to be dried to evaporate into the drier air and this cycle repeats until the system reaches an equilibrium.
Learn more about indicators here:
brainly.com/question/25031970
#SPJ4
1. Draw the molecule that corresponds to each of the names given. a. m-chlorobenzoyl chloride b. methyl butanoate c. butanoic anhydride d. N,N-diethylhexanamide
Answers
a. m-chlorobenzoyl chloride: Cl-C(O)Cl
b. methyl butanoate: CH3-CO-O-CH3
c. butanoic anhydride: (CH3CH2CH2CO)2O
d. N,N-diethylhexanamide: HN(C2H5)2-C6H13-C=O
What are the molecular structures of m-chlorobenzoyl chloride, methyl butanoate, butanoic anhydride, and N,N-diethylhexanamide?a. m-chlorobenzoyl chloride:
Cl
|
C6H4-CO-Cl
b. methyl butanoate:
O
||
CH3-CH2-CH2-COOCH3
c. butanoic anhydride:
O
||
CH3-CH2-CH2-CO-O-CO-CH2-CH2-CH3
d. N,N-diethylhexanamide:
H H H H H H H H
| | | | | | | |
CH3-CH2-C-C-C-C-C-C-N(C2H5)2
| | | | | | |
H H H H H H H
These drawings represent the molecular structures of the given compounds: m-chlorobenzoyl chloride, methyl butanoate, butanoic anhydride, and N,N-diethylhexanamide.
Learn more about molecular structures
brainly.com/question/29857692
#SPJ11