Answers
Explanation:
\({\small{\underline{\bf{\red{answer...}}}}} \\ \\ \)
\(\small\mathfrak\purple{Double \: displacement \: reaction} \\ \\ \small\mathfrak\orange{hope \: it \: helps...}\)
Related Questions
What is thisssssssssss

Answers
Answer:
2Explanation:
As,
Mg + 2HCl ----> MgCl2 + H2
Which makes the equation balanced.
Some atoms have double bonds. Click Remove All, and then add a double bond and two single bonds from the Bonding options.
This molecule now has four bonds, which means the central atom has eight valence electrons. However, these valence electrons are arranged in only three directions around the central atom. Note the bond angles of this molecule.
Remove one of the single-bonded atoms and replace it with a lone pair. How is the remaining bond angle affected by the change?
The bond angle decreases to 109.5°.
The bond angle remains 120°.
The bond angle increases to 180°.
The bond angle remains 109.5°.
Answers
Answer: The bond angle remains 120
Explanation:
A balloon contains 7.36 g of oxygen gas (02). How many oxygen molecules
are in the balloon?
The Periodic Table
O A. 236 molecules
O B. 1.38 x 1023 molecules
O C. 4.43 x 1024 molecules
O D. 15 molecules
Answers
Answer:
15 molecules
Explanation:
A balloon contains 7.36 g of oxygen gas (02). How many oxygen molecules
are in the balloon?
what are two functions of the cilia?
Answers
Answer:
- Proper urine flow by signalling the kidney cells.
- They act as mechanoreceptors or sensory receptors.
Explanation:
Btw I don’t know if this is what you meant
Consider the following chemical equilibrium:
N2 (g) + 3H2 ⇌ 2NH3
Now write an equation below that shows how to calculate Kp from Kc for this reaction at an absolute temperature .
Answers
Answer:
Kp = Kc (RT) ^(-2)
Explanation:
For the reaction;
N2 (g) + 3H2 ⇄ 2NH3(g)
We can write;
Kc = [NH3]^2/[N2] [H2]^3
But
Kp = pNH3^2/pN2 . PH2^3
To convert from Kc to Kp
Kp = Kc (RT) ^Δn
where Δn is the change in number of moles going from reactants
to products.
For this reaction;
Δn = 2- (3+1) = -2
Kp = Kc (RT) ^(-2)
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.
4) A 100g piece of iron at 150°C is emersed in 268.5 g of water at 20°C. the temperature of both iron and water became 25°C. If the specific heat of iron is 0.449 J/g.K, find the specific heat of water?
Answers
Answer:
The specific heat of water would be 4.18 J/gK.
Explanation:
You can realize that iron is losing heat and water is gaining heat. Based on the energy conservation law:
\(Heat\text{ lost by iron = Heat gained by water.}\)We want to find the specific heat of water 'c'. The formula of each heat - lost or gained- is:
\(q=c\cdot m\cdot\Delta T.\)You can see that the change of temperature of iron is from 150 °C to 25 °C and the change of temperature of water is from 20 °C to 25 °C. Remember that the formula of ΔT is:
\(ΔT=|Final\text{ temperature-Initial temperature\mid}\)The change of temperature in celsius will be the same in kelvin. So our initial formula would be:
\(\begin{gathered} Heat\text{ lost by iron=Heat gained by water} \\ c_{iron}\cdot m_{iron}\cdot\Delta T=c_{water}\cdot m_{water}\cdot\Delta T \\ 0.449\text{ }\frac{J}{g\cdot K}\cdot100g\cdot|25-150|K=c_{water}\cdot268.5g\cdot|25-20|K. \end{gathered}\)And finally, we solve for 'c' of water:
\(\begin{gathered} 5612.5\text{ J=c}_{water}\cdot1342.5\text{ g}\cdot K, \\ c_{water}=\frac{5612.5\text{ J}}{1342.5\text{ g}\cdot K}, \\ c_{water}=4.18\frac{J}{g\cdot K}. \end{gathered}\)The specific heat of water would be 4.18 J/gK.
FLAG A nurse is preparing to administer morphine 0.05 mg/kg intermittent IV bolus to a newborn who weighs 3 kg. Available is morphine 0.5 mg/mL injection. How many mL should the nurse administer? (Round the answer to the nearest tenth. Use a leading zero if it applies. Do not use a trailing zero.)
Answers
The quantity of morphine, the nurse should administer is 0.3 ml.
What is morphine?Morphine is a drug which comes under the pain relieving drugs.
The quantity of morphine is 0.05 mg/kg
The weight of the baby is 3 kg.
Available morphine is 0.5 mg/mL.
0.05 x 3 = 0.15 mg
then, 0.15 mg / 0.5 = 0.3
Thus, the quantity of morphine, the nurse should administer is 0.3 ml.
Learn more about morphine
https://brainly.com/question/10665765
#SPJ1
Which amphibian organ has a high blood supply and many folds to increase surface area?
a. heart
b. stomach
c. lungs
d. brain
Answers
Answer:
lungs
Explanation:
if 9.00g grams of gas are enclosed in a 50.00 L vessel at 273.15K and 2.000 atmospheres of pressure , what is the molar mass of the gas? what gas is this?
Answers
Answer: 4.88 g/mol. and helium
Explanation:
To find the molar mass of the gas, we can use the ideal gas law equation which is PV=nRT where:
P = pressure = 2.000 atm
V = volume = 50.00 L
n = number of moles
R = gas constant = 0.08206 L·atm/K·mol
T = temperature = 273.15 K
First, we need to find the number of moles of the gas:
PV = nRT
n = PV/RT
n = (2.000 atm)(50.00 L)/(0.08206 L·atm/K·mol)(273.15 K)
n = 1.844 mol
Now, we can find the molar mass of the gas by dividing its mass by the number of moles:
molar mass = mass/number of moles
mass = 9.00 g
molar mass = 9.00 g/1.844 mol
molar mass = 4.88 g/mol
Therefore, the molar mass of the gas is 4.88 g/mol.
To determine what gas this is, we can compare the molar mass of the gas to the molar masses of known gases. The molar mass of 4.88 g/mol is closest to that of helium (4.00 g/mol). Therefore, this gas is most likely helium.
how many moles are in 22 grams of argon
Answers
Answer:
0.551 moles
Explanation:
To calculate the number of moles in 22 grams of argon, divide the mass by the molar mass:
Number of moles = Mass / Molar mass
Number of moles = 22 g / 39.95 g/mol
Number of moles ≈ 0.551 moles
Therefore, there are approximately 0.551 moles of argon in 22 grams of argon.
what is electrophoresis ?...
Answers
Electrophoresis can be defined as a molecular biology procedure to separate biomolecules depending on the weight and electrical charges.
What is Electrophoresis?Electrophoresis is a molecular biology procedure in which biomolecules such as DNA or proteins can be separated by their electrical charges and weight. For example, DNA has a negative charge thereby it migrates to the positive pole under an electrophoretic field and different DNA molecules can also be separated by their weight in base pairs.
In conclusion, electrophoresis is a technique used in molecular biology laboratories to separate biomolecules depending on the weight and electrical charges.
Learn more about Electrophoresis here:
https://brainly.com/question/6885687
#SPJ1
Consider the equation A(aq) 2B(aq) 3C(aq) 2D(aq). In one experiment, 45.0 mL of 0.050 M A is mixed with 25.0 mL 0.100 M B. At equilibrium the concentration of C is 0.0410 M. Calculate K. g
Answers
Answer:
K = 0.0396
Explanation:
Based on the reaction:
A + 2B ⇄ 3C + 2D
Where equilibrium constant, K, is:
K = [C]³[D]² / [A] [B]²
The initial concentrations of A and B are:
[A]₀ = 0.050M * (45.0mL / 70.0mL) = 0.0321M
[B]₀ = 0.100M * (25.0mL / 70.0mL) = 0.0357M
As [C] = 0.0410M, the molar concentration of D is:
0.0410M * (2mol D / 3mol C) = 0.0273M = [D]
And the concentration of A and B that reacted was:
0.0410M * (2mol B / 3mol C) = 0.0273M B
0.0410M * (1mol A / 3mol C) = 0.0137M A
Equilibrium concentration B and A:
0.0357M - 0.0273M = 0.0084M = [B]
0.0321M - 0.0137M = 0.0184M = [A]
And K is:
K = [0.0410M]³[0.0273M]² / [0.0184M] [0.0084M]²
K = 0.0396Is the ethane molecule more or less polar than water? Why or why not?
Answers
Answer: Less
Explanation:
Ethane is a non-polar molecule while water is a polar molecule
A student named a particular compound 2-ethyl-3-methyl-2-butene. Assuming that the student's choice actually corresponded to the correct distribution of the double bond and the substituents, what is the correct IUPAC name for this compound
Answers
Answer:
2-ethyl-3-methylbut-2-ene
Explanation:
The whole idea of IUPAC nomenclature is to devise a universally accepted system of writing the name of a compound from its structure.
According to IUPAC nomenclature, the root of the compound is the longest carbon chain. The substituents are named in alphabetical order and in such a way as to give each one the lowest number. The position of the functional group is indicated accordingly.
For the compound in question, its correct IUPAC name is 2-ethyl-3-methylbut-2-ene.
Calculate the vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane (C₂H4Br₂, Pº=127 torr)
in 1.80 mol of liquid dibromopropane (C3H6Br2, P=173 torr).
torr
Answers
The vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane the vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane is 164.83 torr.
What is vapor pressure ?The term vapor pressure is defined as the tendency of a material to change into the vapour state, and it increases with temperature.
For calculating mole fraction of C₂H₄Br₂ as follows
X C₂H₄Br₂ = moles of C₂H₄Br₂ / moles of C₂H₄Br₂ + moles of C₃H₆Br₂
= 0.3 / 0.3 + 1.80
= 0.14
For calculating mole fraction of C₃H₆Br₂ as follows:
XC₃H₆Br₂ = moles of C₃H₆Br₂ / moles of C₂H₄Br₂ + moles of C₃H₆Br₂
= 1.80 / 2.1
= 0.85
For calculating total vapor pressure as follows:
P total = [ ( 0.14 × 127) + (0.85 × 173) ]
= 17.78 + 147.05
= 164.83 torr
Thus, The vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane the vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane is 164.83 torr.
To learn more about the vapor pressure, follow the link;
https://brainly.com/question/11864750
#SPJ1
Calculate the pH of an aqueous solution that is 0.200 mol L-1 in NH3 and 0.300 mol L-1 in NH4Cl.
Kb(NH3) = 1.8 x 10-5
Answers
The pH of an aqueous solution that is 0.200 mol/L in NH₃ and 0.300 mol/L in NH₄Cl is 7.9.
We will use Henderson-Hasselbalch equation to calculate the pH. It is given by -
pH = pKa + log [base]
[acid]
We know that,
base is NH₃
acid is NH₄⁺
we will calculate molarity as-
NH₃ : 0.200/2 = 0.1 M
NH₄⁺ : 0.300/2 = 0.15 M
Now, we will find pKa by -
Kw = Ka . Kb
Ka = 1 x 10⁻¹⁴
1.8 x 10⁻⁵
Ka = 5.5 x 10⁻¹⁰
pKa = -logKa
pKa = 9.25
Substituting the values in equation,
pH = 9.25 + log [0.1]
[0.15]
pH = 7.9
Therefore, the pH is found to be 7.9.
To learn more about pH,
brainly.com/question/26728901
#spj1
A 14.579 g sample of CaCl2 was added to 28.016 g of K2CO3 and mixed in water. A 3.558 g yield of CaCO3 was obtained.
What is the limiting reagent?
Answers
Answer:
CaCl3 is the limited reagent
Explanation:
The correct IUPAC name for the structure shown is
A)
ethylmethylamine.
B)
methylamine.
C)
ethylamine.
D)
ethylmethylhydridoamine.

Answers
Answer:
A
Explanation:
it has a methyl group, ethyl group and amine group
The correct IUPAC name for the structure shown in the provided image is "ethylamine." The structure consists of a central nitrogen atom bonded to two carbon atoms.
The correct IUPAC name for the structure shown in the provided image is "ethylamine." The structure consists of a central nitrogen atom bonded to two carbon atoms. According to the IUPAC naming rules, the longest carbon chain is selected as the parent chain, which in this case consists of two carbon atoms. The substituent attached to the parent chain is an ethyl group, denoted as "C2H5". The amine functional group, which consists of the nitrogen atom, is named as "amine". Since there is only one amine group attached to the carbon chain, it is referred to as "ethylamine." Therefore, option C) "ethylamine" is the correct IUPAC name for the given structure.
For more question on IUPAC
https://brainly.com/question/28872356
#SPJ11
Select the correct terms to complete this statement about charged particles.
Like charges attract | repel, and opposite charges attract repel. According to Coulomb's law, as the distance between two charged particles decreases, the force between the particles decreases I increases. As the magnitude of the charges decreases, the force decreases | increases.
Answers
Like charges repel each other, while opposite charges attract each other. This principle is one of the fundamental aspects of electrostatics. According to Coulomb's law, the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
As the distance between two charged particles decreases, the force between them increases. This is because the closer the particles are, the stronger the electric field they create, leading to a stronger force of interaction.
On the other hand, as the magnitude of the charges decreases, the force between the particles also decreases. This is because the force is directly proportional to the product of the charges. If one or both of the charges are smaller, the force they exert on each other will be weaker.
In summary, according to Coulomb's law, decreasing the distance between charged particles increases the force between them, while decreasing the magnitude of the charges decreases the force. This understanding of the relationship between charge, distance, and force is crucial in explaining the behavior of charged particles and the interactions between them.
Know more about Coulomb's law here:
https://brainly.com/question/26892767
#SPJ8
what is Keq for the reaction N2+3H2 = 2NH3 if the equilibrium concentrations are NH3 = 3 M, N2 = 1 M, H2 = 2 M
Answers
The ammonia formation has been 1.125 mol/L.
Keq is defined as the ratio of the mathematical product of the equilibrium concentrations of the species on the right that is multiplied by the concentrations of the chemical products divided by the mathematical product of the equilibrium concentrations of the species on the left.
This is the reaction that makes ammonia from hydrogen and nitrogen. Only one product is produced in this reaction. This reaction is therefore known as a binding reaction. A small Keq Keq < 1 implies a large concentration of reactants at equilibrium. In this case, the reaction drives the formation of reactants. If Keq ≈ 1 it means that there is a significant amount of reactants and products in equilibrium.
Learn more about The equilibrium concentrations here:-https://brainly.com/question/13414142
#SPJ1
Keq is equal to the number 4.5
In Zeff periodicity of valence electron, explain the changes of Al -> Si
Answers
Now, when we compare aluminum (Al) and silicon (Si), we can see that Al is located to the left of Si in the third period of the periodic table.
Aluminum has 3 valence electrons, which are located in the 3p orbital. The effective nuclear charge experienced by these electrons is relatively low because the 3s and 3p electrons shield them from the nucleus. As a result, the valence electrons in Al are relatively easy to remove, making Al a good conductor of electricity.
On the other hand, silicon has 4 valence electrons, which are also in the 3p orbital. However, the effective nuclear charge experienced by these electrons is higher than that experienced by the valence electrons in Al. This is because silicon has more protons in the nucleus, which creates a stronger attractive force on the valence electrons. This makes silicon a poorer conductor of electricity than aluminum.
In summary, the Zeff periodicity of valence electrons explains the changes in the properties of elements across a period. As you move across a period from left to right, the effective nuclear charge (Zeff) experienced by valence electrons increases, making it harder to remove them. This results in changes in the physical and chemical properties of elements such as conductivity, reactivity, and electron affinity.
How is this done I was given the wrong example
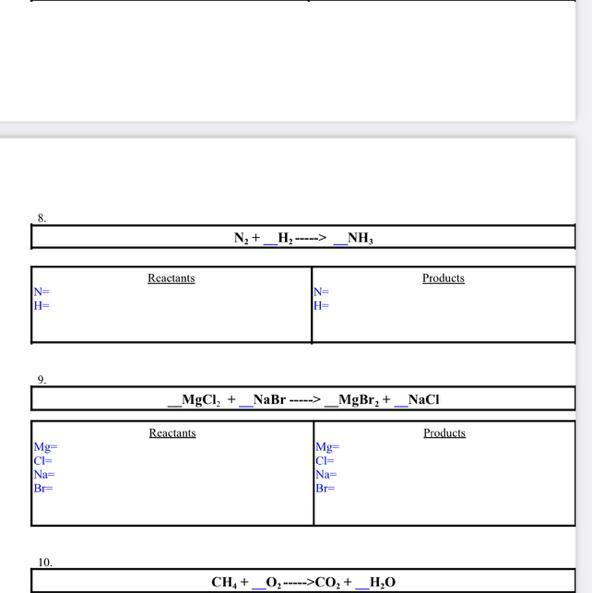
Answers
According to the explanation given in our previous session
8. N2 + 3 H2 -> 2 NH3
Reactants
N = 2
H = 6
Products
N = 2
H = 6
As we can see, both sides now have the same number of elements, 2 Nitrogens and 6 Hydrogens, this is what we need to do in a balancing reaction question
9. MgCl2 + 2 NaBr -> MgBr2 + 2 NaCl
Reactants
Mg = 1
Cl = 2
Na = 2
Br = 2
Products
Mg = 1
Cl = 2
Na = 2
Br = 2
Same number of elements on both sides
What is the triangle G at 294K for the following process at 1.0 atm?

Answers
The ΔG is 3.66 kJ.
To calculate the ΔG of a chemical reaction, it is necessary to use the ΔG formula and replace the values. Remember to convert the J into kJ before the calculation:
\(\begin{gathered} \Delta G=\Delta H-T\cdot\Delta S \\ \Delta G=31.0\frac{kJ}{mol}-294K\cdot0.093\frac{kJ}{\text{mol}\cdot K} \\ \Delta G=3.66\frac{kJ}{mol} \end{gathered}\)So, the ΔG of the reaction is 3.66 kJ.
A scientist measures the mass of two liquids before and after combining them. The mass after combining the liquids is less than the sum of the masses before. How can you explain this?
Answers
The mass after combining the liquids is less than the sum of the masses before because the density of the resulting mixture is a product of the individual densities of the liquids and is less than the density of the denser liquid. Hence, the liquid has a lesser mass.
What is the density of a substance?The density of a substance is the ratio of the mass and the volume of the substance.
Mathematically;
Density = mass / volumeThe density of a substance is a measure of how tightly packed the particles of the substance are.
The density of a substance increases with a decrease in the volume oif a unit mass of a substance.
Learn more about density at: https://brainly.com/question/1354972
#SPJ1
Describe an experiment to show that pressure acts in all directions in liquids.
Answers
We frequently observe kids playing with polythene bags filled with water that have little holes drilled into them at various locations so they can sprinkle water on other kids. Through this experiment, we can say that pressure acts in all directions in liquids.
Liquid's pressureSince both liquids and gases may flow, they are both referred to as fluids. Fluids under rest pressure behave uniformly in all directions.
Weather forecasts can be made using barometers. They track the evolution of atmospheric pressure throughout time.
On weather forecast maps, pressure variations appear as an isobar pattern. Predictions are made using these changes in pressure, and they are fairly accurate when combined with wind observations.
Pressure and depth in liquidsAs you go away from a liquid's surface, pressure rises. for instance: A bucket has three holes that are all the same size. Since there is more pressure at the bucket's bottom, the water spills out more forcefully. Dams are thicker at the bottom for this reason.
Learn more about Pressure here:-
https://brainly.com/question/27984798
#SPJ9
All good experiments should be...
Answers
Inter conversion of glucose and fructose occurs with an eqilibrium constant of 1.0. glicose isomerase catalyzes this reaction. The final concentration of fructose at equilibrim from 40 mM glucose is .
Answers
Inter-conversion of glucose and fructose occurs with an equilibrium constant of 1.0. Glucose isomerase catalyzes this reaction. The final concentration of fructose at equilibrium from 40mM glucose is a. 40 mM.
How to find the final concentration of fructose?Using this formula to find the final concentration of fructose
Final concentration of fructose =Equilibrium from glucose/ Equilibrium constant
Where:
Equilibrium constant = 1.0
Equilibrium from glucose = 40 mM
Let plug in the formula
Final concentration of fructose = 40mM / 1.0
Final concentration of fructose = 40mM
Therefore we can conclude that the correct option is A.
Learn more about Final concentration of fructose here:https://brainly.com/question/14041283
#SPJ1
The complete question is:
Inter-conversion of glucose and fructose occurs with an equilibrium constant of 1.0. Glucose isomerase catalyzes this reaction. The final concentration of fructose at equilibrium from 40mM glucose is
a. 40 mM
b. 20 mM
c. 10 mM
d. 0 mM
CHEM FINAL TOMORROW!!! I'm struggling with a few concepts, if anyone could help explain this to me & how to do it, I'd be very grateful!!!

Answers
Based on the given reaction, the acid-base pairs in this reaction are:
HCO₃⁻ (acid) and NH₃ (base)NH₄⁺ (acid) and CO₃²⁻ (base)What are the acid-base pairs in the given reaction?An acid-base pair refers to a set of two chemical species that are related through the transfer of a proton (H+ ion) during a chemical reaction.
One species acts as an acid by donating a proton, while the other acts as a base by accepting that proton.
In the given reaction:
HCO₃⁻ (aq) + NH₃ (aq) → NH₄⁺ + CO₃²⁻
An acid-base pair can be identified as follows:
HCO₃⁻ (bicarbonate ion) can act as an acid by donating a proton (H⁺), becoming CO₃⁻.
NH₃ (ammonia) can act as a base by accepting a proton (H⁺), becoming NH₄⁺ (ammonium ion).
Learn more about acid-base pairs at: https://brainly.com/question/22514615
#SPJ1
Predict the total pressure in Container C if the initial pressure in Container A was doubled and Container B was reduced by one-half, then mixed in Container C. Show your work.
Answers
The total pressure in Container C is \(5_{P}\)/(\(2_{V}\)). If the initial pressure in Container A was doubled and Container B was reduced by one-half.
To solve this problem, we need to use combined gas law, which relates with pressure, volume, and temperature of a gas;
(P₁V₁)/T₁ = (P₂V₂)/T₂
where P₁ and V₁ are initial pressure and volume, respectively, and T₁ is initial temperature. Similarly, P₂, V₂, and T₂ are inal pressure, volume, and temperature, respectively.
Let's assume that the volume and temperature are constant in all three containers. Therefore, we can simplify the equation to;
P₁/P₂ = V₁/V₂
We can use this equation to solve for the final pressure in Container C.
First, let's calculate the new pressures in Containers A and B;
Container A; the initial pressure was doubled, so P₁ = \(2_{P}\) and V₁ = V (since the volume is constant). Therefore, P₂ = P₁/(V₁/V₂) = \(2_{P}\)/(1/2) = \(4_{P}\).
Container B; the initial pressure was reduced by one-half, so P₁ = P/2 and V₁ = V (since the volume is constant). Therefore, P₂ = P₁/(V₁/V₂) = (P/2)/(1/2) = P.
Now that we have the new pressures in Containers A and B, we can use them to find the total pressure in Container C:
Container C; we are mixing equal volumes of gases from Containers A and B, so the total volume is \(2_{V}\). The total pressure is the sum of the partial pressures of the gases in Containers A and B, which are \(4_{P}\) and P, respectively. Therefore, the total pressure in Container C is:
\(P_{total}\) = (\(4_{P}\) + P)/(\(2_{V}\))
= \(5_{P}\)/(\(2_{V}\))
So, the final pressure in Container C is \(5_{P}\)/(\(2_{V}\)).
To know more about combined gas law here
https://brainly.com/question/13154969
#SPJ1