Which rule for assigning oxidation numbers is correct? Hydrogen is usually â€""1. Oxygen is usually â€""2. A pure group 1 element is 1. A monatomic ion is 0.
Answers
The oxidation number of a specie is determined by certain rules.
The oxidation number of a chemical specie is defined as the charge that the specie is supposed to have as determined by an arbitrary set of rules. We shall now proceed to consider which assignment of oxidation numbers is correct.
1) Hydrogen has a usual oxidation state of +1
2) Oxygen has a usual oxidation state of -2
3) A monoatomic ion can never have an oxidation number of zero because ions are charged.
Learn more about oxidation number: https://brainly.com/question/10079361
Related Questions
IUPAC name for BaSO3
Answers
Answer:
Barium Sulfite
Explanation:
Barium Sulfite
The IUPAC name for BaSO₃ is barium sulfite.
"Ba" stands for barium, which is the chemical symbol for the element with atomic number 56. Barium is an alkaline earth metal and belongs to Group 2 of the periodic table.
"SO₃" stands for sulfite, which is a polyatomic ion composed of one sulfur atom (S) and three oxygen atoms (O). The chemical formula for the sulfite ion is SO₃²⁻. The sulfur atom in the sulfite ion has a +4 oxidation state.
When barium (Ba) reacts with the sulfite ion (SO₃²⁻), they combine to form barium sulfite (BaSO₃). The balanced chemical equation for the reaction is:
Ba²⁺ + SO₃²⁻ → BaSO₃
Barium sulfite is an ionic compound, where the Ba²⁺ ion and the SO₃²⁻ ion are held together by electrostatic attractions (ionic bonds). In its solid form, barium sulfite appears as a white crystalline powder.
Learn more about IUPAC name from the link given below.
https://brainly.com/question/33646537
#SPJ6
PLEASE HELP ME QUICK 22 POINTS RIGHT ANSWERS ONLY!! :)
Will mark brainliest if its right
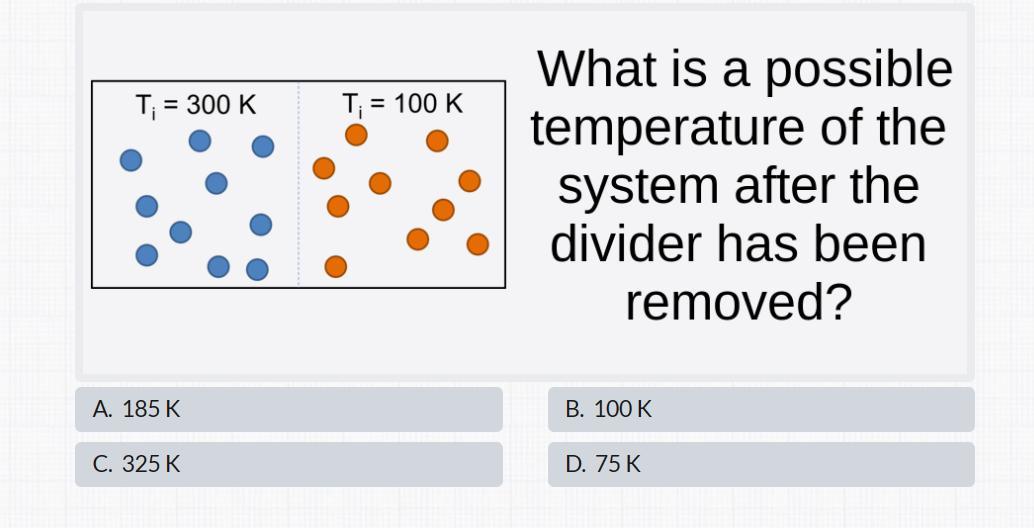
Answers
The correct final temperature after the divider is removed is 200K, amongst the options, the closeset is 185K option A.
The temperature equilibriumTo determine the possible temperature of a system after the divider has been removed, we need to consider the principle of thermal equilibrium and the conservation of energy.
When the divider is removed, the two sides of the system will start to exchange heat until they reach a common temperature. This common temperature is called the final equilibrium temperature.
According to the principle of thermal equilibrium, heat flows from a higher-temperature region to a lower temperature region until the temperatures are equalized.
In this case, the higher-temperature region initially has a temperature of 300K, and the lower-temperature region initially has a temperature of 100K.
To find the final equilibrium temperature, we can use the concept of heat transfer. Heat transfer occurs until the two sides reach the same temperature, so the heat lost by the higher-temperature side must be equal to the heat gained by the lower temperature side.
The heat transferred is given by the equation:
Q = mcΔT
where Q is the heat transferred, m is the mass of the system, c is the specific heat capacity, and ΔT is the change in temperature.
Since the masses and specific heat capacities are not given in the question, we can assume that they are equal on both sides, canceling out these variables.
Therefore, we can calculate the change in temperature:
300K - ΔT = 100K + ΔT
Simplifying the equation:
400K = 2ΔT
ΔT = 200K/2
ΔT = 100K
The change in temperature is 100K. Since the initial lower-temperature side was at 100K, the final equilibrium temperature will be:
Final temperature = 100K + ΔT = 100K + 100K = 200K
Learn more on temperature equilibrium here https://brainly.com/question/9459470
#SPJ1
I don't understand this so if you know please help me

Answers
Explanation:
It's 112-100÷100*100= so 112-100 it's 12÷100 its 0.12 and *100 it's 12%
What happens to metals as they lose thermal energy?
Answers
in which time period did the first animals that could live both in water and on land appear
Answers
To recognize a poisoning pattern, groups of drugs with similar actions, symptoms, and clinical signs are examined. These common signs and symptoms are referred to as the:
a.metabolic pattern.
b.pattern constellation.
c.toxin effect.
d.toxidrome.
Answers
To recognize a poisoning pattern, groups of drugs with similar actions, symptoms, and clinical signs are examined. These common signs and symptoms are referred to as the toxidrome. Hence, the correct option is (d) toxidrome.
What is a toxidrome?
A toxidrome is a group of symptoms and clinical signs that suggest a particular type of poisoning. In the presence of drug-induced toxicities, it is particularly useful for guiding therapeutic decision-making. The clinical signs and symptoms seen in toxidrome reflect the pharmacology of the toxicant, the dose of the toxicant, and the affected organ systems.
Toxidrome pattern
Toxidrome can be divided into five patterns, each of which is associated with a certain type of drug toxicity.
1. Cholinergic toxidrome
2. Anticholinergic toxidrome
3. Sympathomimetic toxidrome
4. Opioid toxidrome
5. Sedative-hypnotic toxidrome
What are the symptoms of a toxidrome?
The following are some of the symptoms that are common in most of the toxidromes:-
Ataxia-Mydriasis-Tachycardia-Tremors-Seizures-Agitation or confusion-Coma or decreased level of consciousness-Respiratory depression or arrest-Bradycardia and hypotension
Toxidrome is a useful tool in drug toxicity management because it can assist clinicians in determining the cause of the poisoning and the best treatment for it.
Learn more about Toxidrome pattern at https://brainly.com/question/30546796
#SPJ11
2. Metals react with water and release hydrogen gas. Explain why non-metals do not release hydrogen gas when reacted with water.
Answers
Answer:
Its because non-metals are unable to break the bond between the H and O ion and cannot reduce hydrogen by donating electrons
What is the mass of 0.55 mole
of magnesium chloride?
Answers
Answer:
SYMBOLS, FORMULAS AND MOLAR MASSES
OBJECTIVES
1. To correctly write and interpret chemical formulas
2. To calculate molecular weights from chemical formulas
3. To calculate moles from grams using chemical formulas
INTRODUCTION
Part I. Symbols and formulas
An element is a homogeneous pure substance made up of identical atoms. All matter is made
up of elements and, since chemistry is the study of matter, it is convenient to use symbols to represent
the elements rather than using the entire name.
By international agreement, specific symbols are assigned to each element (Note: This means
that while names of the elements vary with language, symbols are constant throughout the world.) Each
element is assigned a one- or two-letter symbol. The first letter is capitalized, the second (if there is
one) is not. While this often seems trivial, it is in fact a very important point. For example, in chemical
language Co represents cobalt, which is a metal and an element, while CO represents carbon monoxide,
a compound which is a colorless, odorless gas! Even when there is not an obvious correspondence,
for instance "MN", it can cause confusion. Do you mean the element manganese? Did you forget a
letter and mean something else? Are you using "M" to represent something else entirely? Chemists
sometimes use "M" to represent any metal. It is well worth the trouble to memorize the symbols for
common elements.
Since compounds consist of elements, the chemical formulas of compounds also consist of
elements with subscripts used to denote the number of atoms per molecule. If there is no subscript, it is
implied that there is one of that kind of atom. Ones never appear in chemical formulas. Not only do
subscripts denote ratios of atoms, they also denote the ratio of moles of element to one mole of
compound. Parentheses can be used to show groups of atoms, with the subscripts showing how many
groups there are. Parentheses are not used if there is only one group.
Examples: For one mole of the following compounds, how many moles of each element are
present?
MgCl2 1 mole Mg, 2 moles Cl
Mg(NO3)2 1 mole Mg, 2 moles N, 6 moles O
NaNO3 1 mole Na, 1 mole N, 3 mole O
AgCl 1 mole Ag, 1 mole ClPart II. Molar Masses
Each atom has a different size and therefore a different mass. The relative masses of each
element can be found on the periodic table. For example, one atom of magnesium weighs 24.31 amu
(atomic mass units). However, one mole of magnesium weighs 24.31 g. (Moles were planned that
way!) Since one mole of MgCl2 consists of one mole of magnesium and two moles of chlorine, the
mass of one mole of MgCl2 must be the sum of the masses of the elements. The mass of one mole of a
substance is called the molar mass or molecular weight.
Examples: What is the molar mass of the following compounds?
MgCl2 24.31 + 2(35.45) = 95.21 g/mol
Mg(NO3)2 24.31 + 2(14.01) + 6(16.00) = 148.33 g/mol
NaNO3 23.00 + 14.01 + 3(16.00) = 85.01 g/mol
AgCl 107.9 + 35.45 = 143.4 g/mol
(Note: Yes! You DO have to count significant figures when calculating molecular weight/molar
mass. However, the number of significant figures may vary depending on which periodic table you use.)
Chemists are generally interested in number of moles. Unfortunately, it is impossible to measure
moles directly. However, masses are easily measured, and if the chemical formula of the compound is
known, the molar mass can be used to determine the number of moles. The molar mass is defined as:
molar mass = grams/moles = g/mol (1)
Moles may be calculated by using molar mass as a conversion factor in dimensional analysis where
molar mass in grams = 1 (exactly) mole of compound (2)
This method is used in multi-step calculations. For example, if 0.873 g of MgCl2 is weighed out, it
is 9.17 x 10-3
moles.
1 mole
0.873g x 95.21 g = 9.17 x 10-3
mol MgCl2 (3)
However, 0.873 g of AgCl is only 6.09 x 10-3
mol.
1 mole
0.873g x 143.4 g = 6.09 x 10-3
mol AgCl (4)Molar mass may also be used to relate moles to grams. For example, 0.158 mol of MgCl2 is 15.2 g.
0.158 mol x 95.21 g = 15.2 g MgCl2 (5)
1 mol
Percent is used to express parts per one hundred. Usually in chemistry, it refers to
g of species of interest x 100 = % (6)
g of whole thing
Example: For the % Mg in MgCl2: In one mole of MgCl2, there are 24.31 g of Mg (molar mass of Mg,
the part we are interested in) and 95.21 g of MgCl2 (the whole thing), so %Mg in MgCl2 is
(24.31/95.21) x 100 = 25.53% Mg (7)
PROCEDURE
Work individually.
The formula for calcium phosphate is Ca3(PO4)2. Weigh about 2 g of calcium phosphate to the
nearest 0.001 g. In other words, you do not have to have exactly 2.000g, but you must know the
weight you have exactly. Acceptable results include but are not limited to: 1.985g , 2.035g, 2.314g
etc.
Be sure to report all results with the correct number of significant figures and appropriate units!
The pH is given for three solutions. Calculate (H+] and [OH-] in each solution.
b. pH = 11.05
Plss show work
Answers
Oh=1.12*10^3
Calculate the volume of gas, in L, occupied by 1.74 moles of SF6 (g) at 273 K and 1 atm
Answers
The volume of gas is 3,949.31 litre, occupied by 1.74 moles of SF6 (g) at 273 K and 1 atm.
What is an ideal gas law ?The ideal gas law is also called as perfect gas law. It is the relation between the pressure P, volume V, and temperature T of a gas.
Given:
Volume of gas = ?
Number of moles (n) = 1.74 moles
Temperature (T) = 273K
Pressure (P) = 1 atm
R (gas constant) = 8.314
By an ideal gas equation
PV = nRT
By putting above value in ideal gas equation, we get
1 × V = 1.74 × 8.314 × 273
V = 3,949.31 litre
Thus, The volume of gas, in L is 3,949.31 litre, occupied by 1.74 moles of SF6 (g) at 273 K and 1 atm.
To learn more about an ideal gas law, follow the link;
https://brainly.com/question/4147359
#SPJ1
4. Public water supplies are unsafe in some parts of the world because the water
and
may carry
Answers
Answer:
In many parts of the world, water taken directly from wells or public supplies is not safe to drink because it may carry waterborne parasites or other diseases. The water can be sterilized by boiling it, but fuel is needed to do so.
Explanation:
Answer: Public water supplies are unsafe in some parts of the world because the water may carry bacteria.
Explanation:
what is the purpose of an auxiliary complexing agent?
Answers
Answer:
prevent hydroxide formation
Explanation:
Ca + Cl - > 2CaCl3
the two is a subscript, coefficient, reactant, or superscript?
Answers
plz solve the question and send the answer
I will give u branist, follow u ,rate u 5 star and also give u like ,plz help me

Answers
Answer:
64g of \(\bold{CH_{3}OH}\dashrightarrow\)44.8L
vapour density of \(CH_{3}3OH=\frac{mass}{volume}\) of \(\bold{CH_{3}OH}\)
=64/44.8=10/7=1.43 g/l
Vapour density of \(\bold{CH_{3}OH}\)=1.43g/l
64g of \(\bold{CH_{3}OH => 44.8L }\)
vapour density of \(\small{\sf{CH_{3}3OH=\frac{mass}{volume} of } \bold{CH_{3}OH}}\)
=64/44.8=10/7=1.43 g/l
Vapour density of \(\bold{CH_{3}OH = 1.43 g/L}\)
minerals such as quartz, that break along jagged edges are said to have
Answers
Answer:
Minerals such as quartz, that break along jagged edges are said to have hackly fracture.
Cleavage is the tendency of a mineral to break along smooth, flat surfaces. Hackly fracture is the tendency of a mineral to break along irregular, jagged surfaces. Quartz is a mineral that has hackly fracture. It breaks along irregular, jagged surfaces because its atoms are tightly bonded together. When quartz is struck, the atoms are unable to slide past each other, so the mineral breaks along irregular, jagged surfaces.
why is the mass of kcl recovered less than the starting mass of khco3
Answers
The mass of KCl recovered can be less than the starting mass of KHCO3 due to several factors, such as:
1. Incomplete conversion: The reaction between KHCO3 and HCl to form KCl involves a stoichiometric ratio. If the reaction is not driven to completion or if there are side reactions or competing reactions, it may result in an incomplete conversion of KHCO3 to KCl. This would lead to a lower mass of KCl recovered compared to the starting mass of KHCO3.
2. Losses during the process: During the reaction and subsequent processes like filtration or drying, some of the product (KCl) or reactant (KHCO3) may be lost. Losses can occur due to physical losses like splattering or spilling, or chemical losses like volatilization of certain compounds.
3. Impurities or contaminants: The starting KHCO3 may contain impurities or contaminants that do not convert to KCl during the reaction. These impurities or contaminants can remain in the reaction mixture or be lost during subsequent purification steps, leading to a difference in the mass of KCl recovered.
It is important to ensure proper reaction conditions, efficient conversion, and minimize losses during handling and purification to achieve a higher recovery of the desired product.
To know more about mass of KCl refer here
https://brainly.com/question/17489670#
#SPJ11
Which system controls organs in times of stress?
Answers
Answer:
The autonomic nervous system has a direct role in physical response to stress and is divided into the sympathetic nervous system (SNS), and the parasympathetic nervous system (PNS). When the body is stressed, the SNS contributes to what is known as the “fight or flight” response.
Explanation:
I NEED HELP ASAP RIGHT NOW PLEASE

Answers
Answer:
I think it's C or D Sorry if I'm Wrong
Explanation:
it just makes the most sense to me
Which of the diagrams below shows an atom that is not a form of hydrogen?

Answers
Answer:
I believe is the npp i kinda forgot how to do these lol
Diagram with npp type shows an atom that is not a form of hydrogen.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/30898688
#SPJ2
this is science
7. Magma that hardens in a volcano's pipe forms a
A. Volcanic neck
B. Sill
C. Volcanic crater
D. Hot spot
Answers
Help me out with this question please

Answers
Explanation: To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. They do this by hydrating the ions. ... When you place an ionic substance in water, the water molecules attract the positive and negative ions from the crystal.
What is the name of NoCk4? Explain how you determined the bond type and the steps you used to determine the naming convention for the compound.
Answers
What does the statement mean: "You are as safe as the least safe person in the laboratory"?
Answers
Based on laboratory safety protocols, statement "You are as safe as the least safe person in the laboratory" means that the person is not completely safe.
What is safety?Safety refers to the state where an individual or a person is free from danger or harm.
Safety is essential to all individuals in order to ensure that the individual is thriving.
There are several forms of safety that is related to an individual being safe.
The forms of safety may include:
Workplace or occupational safetySafety in the homeSafety at schoolLaboratory safety.In order to ensure that an individual is safe, safety procedures and protocols are put in place which people must follow in order to be safe in their environment.
In the laboratory, because of the presence of hazardous chemical, it is important that safety protocols are followed. Some safety protocols in the laboratory include:
always wear lab coatswear safety gogglesdo not eat in the laboratoryTherefore, the statement "You are as safe as the least safe person in the laboratory" means that the individual is not safe.
Learn more about laboratory safety at: https://brainly.com/question/17994387
#SPJ1
In certain species of fish, scale color can either be blue, yellow, or blue and yellow striped. If a blue fish and a blue/yellow striped fish mate, what color scales could their offspring have?
Answers
Answer:
Blue scale or blue/yellow stripe scale
Explanation:
Let us assume that the blue color is represented by B allele while the yellow color is represented by Y allele. The two alleles are equally dominant on one another, hence, BY would represent blue and yellow stripe gene.
Blue scale - BB
Yellow scale - YY
Blue/yellow striped scale - BY
Crossing BB and BY
BB x BY
BB BY BB BY
Hence, their offspring is either BB (blue scale) or BY (blue/yellow stripe scale).
What is a solubility curve and what does it measure?
Answers
Answer:
a thing that does things
Explanation:
Calculate the percent composition of O in K3PO4
Answers
The percent composition of oxygen in tripotassium phosphate is 30.15%.
What is percent composition?Percent composition is defined as a convenient way to record concentration of solution.It is a expression which relates mass of an element to compound as,mass of element/mass of compound ×100.
In the given compound of tripotassium phosphate the molar mass is 212.27 g/mole, while that of oxygen is 16×4=64 g/mole.Thus, the percent composition of oxygen in tripotassium phosphate is 64/212.27×100=30.15%.
Thus, the percent composition of oxygen in tripotassium phosphate is 30.15%.
Learn more about percent composition,here:
https://brainly.com/question/20527749
#SPJ1
Advanced Study Assignment (ASA) The visible spectrum of light links the wavelength of a photon of light to the color our eyes perceive. The visible spectrum is given below: 1. Arrange the following wavelengths in order of increasing energy:45.5 nm,1050 nm,325 nm,715 nm,450 nmLeast energyâ<_<1<1Most energy 2. What is the wavelength of light emitted when an electron in a hydrogen atom transitions from then=5to then=3level? The following energy levels were recorded for a sodium atom: Calculate the energy of a photon needed to cause an electron in the 3s orbital to be excited to the 3p orbital.
Answers
The visible spectrum of light is an important tool used in understanding the behavior of light. The wavelength of a photon determines the color that our eyes perceive. The shorter the wavelength of a photon, the higher its energy.
The correct order from least energy to most energy is: 1050 nm, 715 nm, 450 nm, 325 nm, 45.5 nm. This is because the wavelength of 1050 nm has the longest wavelength and therefore the lowest energy, while the wavelength of 45.5 nm has the shortest wavelength and the highest energy.For question 2, we need to determine the wavelength of light emitted when an electron in a hydrogen atom transitions from n=5 to n=3 level. The energy difference between these two levels is calculated using the Rydberg formula: E = (Rh / n^2) x (1/3^2 - 1/5^2) = 0.84 eV. The energy of the photon emitted during this transition is equal to the energy difference between the two levels. Therefore, the wavelength of the photon emitted is given by the formula: λ = hc / E = 1.48 x 10^-6 m. For the final question, we need to calculate the energy of a photon needed to cause an electron in the 3s orbital of a sodium atom to be excited to the 3p orbital. The energy difference between these two levels is equal to the energy of the photon absorbed during the transition. This energy is given by the formula: E = (Rh / n^2) x (1/3^2 - 1/2^2) = 2.10 eV. Therefore, the energy of the photon needed is 2.10 eV. Using the formula λ = hc / E, we can calculate the wavelength of the photon to be 590 nm. In summary, understanding the relationship between energy and wavelength is essential to answering questions related to the visible spectrum of light and hydrogen atom transitions.
Learn more about electron here-
https://brainly.com/question/1255220
#SPJ11
Calculate the mass of sulfur that must react to produce 9. 30 L of sulfur dioxide (SO,) at
740 mmHg and 125°C
Answers
6.07 g of sulfur must react to produce 9.30 L of sulfur dioxide at 740 mm Hg and 125°C.
The given conditions of the reaction can be used to find the number of moles of sulfur dioxide using the ideal gas law, PV = nRT, where P = 740 mmHg, V = 9.30 L, T = 125 + 273 = 398 K, and R = 0.0821 L atm/mol K.
First, we need to convert pressure to atm. 1 atm = 760 mmHg, therefore, P = 740 mmHg/760 mmHg/atm = 0.974 atm
Using the ideal gas law, we have:
0.974 atm × 9.30 L = n × 0.0821 L atm/mol K × 398 K
n = 0.377 mol
The balanced equation for the reaction is:
S + 2O2 → 2SO2
For every 2 moles of SO2 produced, 1 mole of sulfur is required. Therefore, the moles of sulfur required to produce 0.377 mol of SO2 is 0.377/2 = 0.1885 mol.
The molar mass of sulfur is 32.07 g/mol, so the mass of sulfur required is:
0.1885 mol × 32.07 g/mol = 6.07 g
To learn more about ideal gas law, here
https://brainly.com/question/28257995
#SPJ4
Select the curve that is produced by adding hydrochloric acid to 25 cm3 of sodium hydroxide.A,B,C or D

Answers
B
The sodium hydroxide (NaOH) solution is a basic solution, so the pH of that solution should be close to 14
then when adding hydrochloric acid (HCl) we start to neutralice the solution, meaning the pH must sift slowly to lower pH.
Assuming both solutions have similar concentration the pH shall shift form basic (above 7) to acid pH (below 7). Until now both B and D images agreed with the explanation given. To chose between them we need to remember that HCl is a very strong acid, which means that in solution will get to very acid solutions (very low pH values) which leaves only B as possible answer
PLEASE HELP FAST! What's the IUPAC name of the compound shown?
Answers
Regarding the location of the double bond that takes priority in the provided structure, it is 3 - bromo - 1 - chlorocyclohex - 1 -ene. In short, the name of the compound is written out first.
Followed by the base name (which is derived from the number of carbons in the parent chain) and the substituents in alphabetical order. Between numbers and letters are separated by dashes and commas, respectively. The name has no spaces. The International Union of Pure and Applied Chemistry (IUPAC) recommends using the IUPAC nomenclature of organic chemistry when naming organic chemical compounds in chemical nomenclature.
To learn more about carbons, click here.
https://brainly.com/question/22530423
#SPJ4