Which statement below is TRUE about COMPOUNDS? *
a) Compounds are made up of the same type of atoms.
b) Compounds are made up of only 1 atom.
d) Compounds are made up of different atoms stuck together.
d) Compounds can only contain 2 atoms.

Answers
Related Questions
when potassium chloride (kcl) is dissolved in water the temperature decreases. fill in the blanks to explain why this occurs. the energy required to separate the k and cl- and to separate the water molecules is -- the energy produced by the attractions between the k and cl-
Answers
When potassium chloride (KCl) is dissolved in water, the temperature decreases because the energy required to separate the potassium (K) and chloride (Cl-) ions and the energy required to separate the water molecules is greater than the energy produced by the attractions between the K and Cl- ions.
When KCl dissolves in water, the K and Cl- ions are surrounded by water molecules. The process of dissolving involves breaking the ionic bonds between the K and Cl- ions and the formation of new ion-dipole interactions between the ions and water molecules. To separate the K and Cl- ions, energy must be supplied to overcome the attractive forces between them. Additionally, to separate the water molecules, energy is required to disrupt the intermolecular hydrogen bonding between the water molecules. The energy produced by the attractions between the K and Cl- ions is not sufficient to compensate for the energy required to break these bonds. As a result, energy is absorbed from the surroundings, which leads to a decrease in temperature. The endothermic process of dissolving KCl in water, which requires more energy input than energy released, causes the temperature of the solution to decrease.
for more questions on temperature
https://brainly.com/question/4735135
#SPJ8
A. Calculate E, AGº, and K for the spontaneous reaction between the species in the two appropriate half reactions. The standard reduction potentials are listed as follows: Include the balanced redox reaction as part of your answer. (12 pts) Agt te --> Ag (8) Eº=0.80 V Fe3+ + 3e ----> Fe(s) Eo = 0.77 V 4B. Draw a galvanic cell for the redox reaction taking place in problem #4A. The reduction potentials are listed as follows: Agt + e ---> Ag (s) Eº=0.80 V Fe3+ + 3e ----> Fe(s) E=0.77 V Label the anode, cathode, the direction of electron flow, and migration of Nat and NO3" ions in the salt bridge toward the appropriate electrodes. In addition, write out the proper shorthand or line notation for this galvanic cell (12 pts) AG° = -NFE° F= 9.65 x 104 C/mol e- E° =(0.0592/n)logK 1 V = 1 J/C 1 Amp = 1 C/s AGỌ=-RTINK AG®rxn-ZAG (products)-EAGL (reactants) ASørx-S (products)-S°(reactants) AGO=AH-TASO AE =q+w S=kblnW
Answers
The balanced redox reaction for the spontaneous reaction between AgTe and Fe3+ is:
AgTe + Fe3+ → Ag + Fe2+ + Te
The corresponding half-reactions with their standard reduction potentials are:
Ag+ + e- → Ag (Eº = 0.80 V)
Fe3+ + e- → Fe2+ (Eº = 0.77 V)
To calculate the overall standard cell potential (Eº), we need to subtract the reduction potential of the anode (Fe3+ → Fe2+) from the reduction potential of the cathode (Ag+ → Ag):
Eºcell = Eºcathode - Eºanode
Eºcell = 0.80 V - 0.77 V
Eºcell = 0.03 V
The standard free energy change (ΔGº) can be calculated from the standard cell potential (Eº) using the equation:
ΔGº = -nFEº
where n is the number of electrons transferred, F is the Faraday constant (9.65 x 10^4 C/mol e-), and Eº is the standard cell potential.
In this case, n = 1 (one electron is transferred), so:
ΔGº = -1 x (9.65 x 10^4 C/mol e-) x (0.03 V)
ΔGº = -2.89 kJ/mol
The equilibrium constant (K) can be calculated from the standard free energy change (ΔGº) using the equation:
ΔGº = -RT ln K
where R is the gas constant (8.31 J/mol K), T is the temperature in Kelvin (298 K), and ln is the natural logarithm.
Substituting the values, we get:
-2.89 x 10^3 J/mol = -(8.31 J/mol K) x (298 K) x ln K
Solving for K, we get:
K = 1.47 x 10^16
Therefore, the standard cell potential (Eº) is 0.03 V, the standard free energy change (ΔGº) is -2.89 kJ/mol, and the equilibrium constant (K) is 1.47 x 10^16.
B)
The galvanic cell for the redox reaction taking place in problem 4A can be represented as:
Anode: Fe(s) | Fe2+(aq) || Ag+(aq) | Ag(s) : Cathode
For more similar questions on electrochemistry https://brainly.com/question/17066890
#SPJ11
The percent composition of nh42c2o4
Answers
Answer: Percent composition by element
Element Symbol Mass Percent
Hydrogen H 6.498%
Carbon C 19.357%
Nitrogen N 22.574%
Oxygen O 51.571%
HOPE THIS HELPS
A cell with a 10% salt concentration inside it is placed into a solution. The cell begins to shrink. What is a possible concentration of salt outside the cell
Answers
Given what we know, we can confirm that the concentration of salt outside the cell was above 10%.
How can we know the external salt concentration?This has to do with osmosis. Osmosis is the tendency of water to move towards a place with a higher concentration of solute. This is because of water's role as a solvent. The cell in this case shrinks because the water leaves the cell to dissolve the outside salt concentration, which tells us that it must be higher than 10%.
Therefore, we can confirm that the cell shrinks because water leaves the cell to dissolve the outside salt concentration, meaning that the outside concentration of salt is higher than 10%.
To learn more about osmosis visit:
https://brainly.com/question/21395644?referrer=searchResults
When bubbles form in a liquid, which physical change is happening?
A. condensing
B. boiling
C. freezing
D. melting
Answers
Bubbles often mean a substance is turning into a gas form. Because the bubbles are shown it means the liquid is turning into a gas and that is done through boiling. (Water is heated up until it becomes a gaseous vapor)
Add distilled water to the beaker until the volume
totals 15 mL.
Record the amount of cornstarch that dissolved.
all
about half
none
20
25 ml
15
10
5
Intro
Answers
Answer:
The correct answer on edge for 2021 is C. None.
Explanation:
We know this is correct because the solution is heterogenous after it has been stirred: the substance is not soluble in water.

The solution is heterogeneous after it has been stirred. The cornstarch is not soluble in water. Therefore, option C is correct.
What is the solution?Any mixture of one or more solutes that have been dissolved in a solvent is referred to as a solution. To create a homogenous mixture, a solute must dissolve in a solvent. To create a homogenous mixture, a solute must dissolve in a solvent.
Whereas homogenous mixtures seem consistent throughout, heterogeneous mixtures have clearly discernible components. A solution, which can be a solid, liquid, or gas, is the most typical kind of homogenous mixture.
No matter how you sample a homogenous solution, it often has the same characteristics. Sources of water, saline solution, certain metals, and bitumen are homogenous mixes. Examples of heterogeneous combinations are chicken noodle soup, sand, and oil, and water.
Thus, option C is correct.
To learn more about the solution, follow the link:
https://brainly.com/question/30665317
#SPJ7
as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
How many kernel electrons in Ne?
Answers
Answer:
I am pretty sure it has 10 kernel electrons!?!?!?
Hope this helps!!!
Have a great day!!! :)
how many molecules are in 80 grams of Bromine
Answers
Answer:
\(6.029 \times 10^{23} molecules\)

Mr. Wang works in a recycling center. Recyclable materials arrive at the center mixed. Workers use magnets to separate steel cans from other items. Which two statements are true about the force between a steel can and a magnet?
Answers
Answer:
Option 3, The attraction between the can and the magnet is a pull.
Explanation:
The complete question is
Mr. Wang works in a recycling center. Recyclable materials arrive at the
center mixed together. Workers use magnets to separate steel cans from
other items. Which two statements are true about the force between a steel can and a magnet?
1 Gravity pushes the can toward the magnet.
2 The force between the can and the magnet is a noncontact force.
3 The attraction between the can and the magnet is a pull.
4 The attraction between the can and the magnet is a push
Solution
The force exerted by magnet on steel is the pull force. In magnets unlike poles attract each other (pull force) while the like poles repel (push force). Now, the steel or any ferrous object in the garbage when experience magnetic field develop magnetic field of their own in such a way that their north always faces the south of the external magnet or vice versa.
Hence, the force between a steel can and a magnet is pull force
Given the balanced equation representing a reaction:
Ni(s) + 2HCl(aq) - NiCl(aq) + H2(9)
In this reaction, each Ni atom
1.
loses 1 electron
2.
loses 2 electrons
لما
gains 1 electron
4
gains 2 electrons
Submit
Answers
Answer: loses 2 electrons
Explanation:
Ni Atom loses 2 electrons , Option 2 is the correct answer.
What is an Electron ?Electron is a subatomic particle (denoted by the symbol e⁻)
or whose electric charge is negative one elementary charge.
Electrons belong to the first generation of the lepton particle family and are generally thought to be elementary particles because they have no known components or substructure.
The electron has a mass that is approximately 1/1836 that of the proton.
Ni(s) + 2HCl(aq) - NiCl(aq) + H₂(g)
This is an oxidation-reduction (redox) reaction:
2 H⁺ + 2 e⁻ → 2 H (reduction)
Ni - 2 e⁻ → Ni ²⁺ (oxidation)
HCl is an oxidizing agent, Ni is a reducing agent.
Therefore Ni Atom loses 2 electrons , Option 2 is the correct answer.
To know more about Electron
https://brainly.com/question/12001116
#SPJ2
Which section of the reaction represents the reactants

Answers
how to calculate the error in a standard solution chemistry 14cl
Answers
To calculate the error in a standard solution of chemistry 14cl, you need to first measure the exact mass of the solution and subtract the amount you initially put in the solution. The result is the error.
For example, if you put 10 g of chemistry 14cl into a solution and measure the exact mass to be 10.4 g, the error would be 0.4 g.
To calculate the error in a standard solution in chemistry, you need to follow these steps:
1. Calculate the amount of substance you should have in your solution. This is typically given in the question, or you can calculate it using the formula n = cV, where n is the amount of substance, c is the concentration, and V is the volume.
2. Calculate the amount of substance you actually have in your solution. This can be done by weighing the solution and using the formula m = nM, where m is the mass, n is the amount of substance, and M is the molar mass.
3. Calculate the difference between the amount of substance you should have and the amount you actually have. This is the error in your solution.
4. Divide the error by the amount of substance you should have to get the percent error. This is typically reported as a percentage.
For example, if you were supposed to have 14cl of a substance in your solution, but you actually have 13.8cl, the error would be 0.2cl. The percent error would be (0.2/14) * 100 = 1.43%.
So, the error in a standard solution in chemistry can be calculated by finding the difference between the amount of substance you should have and the amount you actually have, and then dividing by the amount you should have to get the percent error.
For more questions related to standard solution.
https://brainly.com/question/18720754
#SPJ11
look at the screenshot ;)

Answers
This is not the best way to organize a periodic table because two elements might have similar atomic mass.
What is periodic table?Periodic table is a chart in which arrangement of chemical elements are done.
In the early periodic table elements are arranged on the basis of their atomic masses, while after sometime Moseley arranged the periodic table on the basis of atomic number as he proposed that properties of an element is justified on the basis of number if electrons.
And mass of two substances may be same so it is difficult to differentiate between them.
Hence, two elements might have similar atomic mass.
To know more about periodic table, visit the below link:
https://brainly.com/question/14514242
#SPJ1
what is the formula managers use to calculate a foodservice operation’s total expense percentage?
Answers
The formula managers use to calculate a food service operation's total expense percentage is to divide the total cost of food by total sales.
The resulting amount should then be multiplied by 100 to get the percentage. A food service operation's total expense percentage is the percentage of its total sales that are spent on food.
The formula to calculate the food service operation's total expense percentage is:
Expense percentage = (Total cost of food / Total sales) * 100
This formula gives the total amount or the total expense percentage which a food service operation spends on its food to generate its total sales or revenue.
In this calculation, the total cost of food includes all expenses associated with purchasing, preparing, and serving the food, such as ingredients, labor, and supplies.
The calculation of the food service operation's total expense percentage is critical for the management and profitability of the business. It helps to determine the actual cost of the food and the operation's overall profitability.
By comparing the expense percentage of the business with industry standards and previous performance, management can make informed decisions to control costs, increase revenue, and maximize profits.
Learn more about operations here:
https://brainly.com/question/30010720
#SPJ11
Help please!!!
Is tap water electrolytes or not?
Is ethyl alcohol solution electrolytes or not?
Is pure sodium chloride electrolytes or not?
Answers
Answer:
Tap Water: Tap water has Electrolytes
Ethyl alcohol solution: Ethyl alcohol solution has no electrolytes
Pure sodium chloride: sodium chloride has electrolytes.
Explanation:
Hope that helps
Explanation:
1, tap has water electrolytes
2, Ethyl alcohol soultion does not have electrolytes
3, Pure Sodium Chloride does have Electrolytes
How many moles of Nitrogen (N2), are
needed to react with 6 moles of Hydrogen
(H2)?
N2 + 3H2 -> 2NH3
Answers
2 mol N₂
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Reading a Periodic TableStoichiometry
Using Dimensional AnalysisExplanation:Step 1: Define
[RxN - Balanced] N₂ + 3H₂ → 2NH₃
[Given] 6 mol H₂
Step 2: Identify Conversions
[RxN] 3 mol H₂ → 1 mol N₂
Step 3: Stoichiometry
Set up: \(\displaystyle 6 \ mol \ H_2(\frac{1 \ mol \ N_2}{3 \ mol \ H_2})\)Divide: \(\displaystyle 2 \ mol \ N_2\)When a younger rock cuts through an older rock it is known as a(n)
Answers
Explanation: when a rock cuts into another rock that’s older than it is, it is known as a fault
How do we seperate a mixture of water and sugar
Answers
Answer:
The easiest way to separate a mixture of sugar and water is to use distillation, a process that separates substances based on their different boiling points. 《☆☆☆☆☆》
Explanation:
Hope it helps JOIN 《Æ §QŮÅĐ》
the oxidant in a reaction that removes 2 electrons and 2 protons from glyceraldehyde 3-phosphate is called?
Answers
The oxidant in a reaction that removes 2 electrons and 2 protons from glyceraldehyde 3-phosphate is called a "reducing agent" or an "electron acceptor". It is responsible for the oxidation of the glyceraldehyde 3-phosphate.
A substance that loses electrons to other substances in a redox reaction and gets oxidized to a higher valency state is called a reducing agent.
In glycolysis, during oxidation electrons are removed by NAD+ which is then converted into NADH2.
Oxidation of glyceraldehyde 3-phosphate into 1,3-bis phosphoglycerate leads to the production of 2 NADH2 molecules.
To know more about Sociologists, refer here:
https://brainly.com/question/29947859
#SPJ11
Question is in the photo

Answers
In the heating and cooling curves below, identify the letter in the diagram diagram that corresponds to each of the listed processes in the table
I’m so confused if anyone could help (and explain as if I’m a 3 yr old) that would be helpful
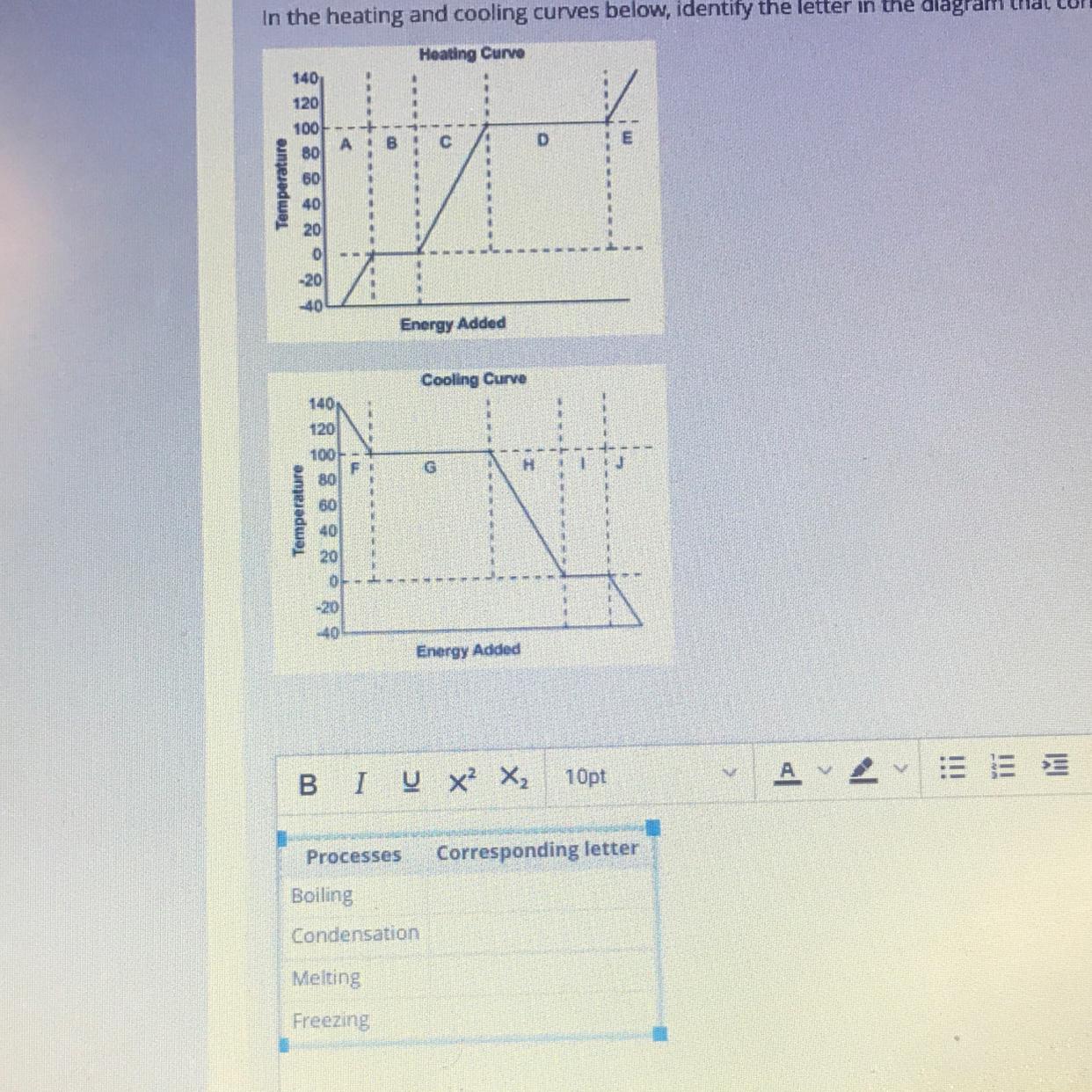
Answers
Answer:
Test for the first one is the best for
Please help now ASAP lpleaseee helppp

Answers
Phosphorous at 18.9%. According to analysis, sodium, phosphorus, and oxygen make up 42.1%, 18.9%, and 39% of sodium phosphate, respectively.
What is the short definition of percentage composition?The ratio of the amounts of each element to the sum of the individual element amounts present in the compound, multiplied by 100, is the definition of the percentage of a given compound. In this instance, the quantity is expressed in grams of the constituent ingredients.
What distinguishes mass percent from percent composition?The proportional mass of each component of a compound is known as the percent composition. The mass percent of a compound is the weight of an element expressed as a share of the complex's overall mass. For instance, a molecule of water has a structure of one oxygen atom and two hydrogen atoms.
To know more about percentage composition visit:
https://brainly.com/question/11856940
#SPJ1
COULD SOMEONE PLEASE GIVE ME THE ANSWER FOR THIS NO BOTS PLEASEEE TODAY IS THE LAST DAY FOR MISSING WORK!!!!

Answers
Answer:
:D
Explanation:
8. D. All of the above
9. C. Mechanical weathering
10. D. All of the above
Answer:
8. D
9. A
10. D
The molecule beryllium chloride has one beryllium atom, a metal, and two chlorine atoms, nonmetals. what kind of bond will they form?(1 point) responses beryllium and chlorine will form an ionic bond where one beryllium atom will donate electrons to two chlorine atoms. beryllium and chlorine will form an ionic bond where one beryllium atom will donate electrons to two chlorine atoms. beryllium and chlorine will form an ionic bond where two chlorine atoms will donate electrons to one beryllium atom. beryllium and chlorine will form an ionic bond where two chlorine atoms will donate electrons to one beryllium atom. beryllium and chlorine will form a covalent bond where one beryllium atom will share electrons with two chlorine atoms. beryllium and chlorine will form a covalent bond where one beryllium atom will share electrons with two chlorine atoms. beryllium and chlorine will form a covalent bond where two chlorine atoms will share electrons with one beryllium atom.
Answers
The kind of bond they will form is an ionic bond where one beryllium atom will donate electrons to two chlorine atoms (option A).
What is an ionic bond?Ionic bond is a type of chemical bond where two atoms or molecules are connected to each other by electrostatic attraction.
Ionic bonds are formed by atoms with opposite charges i.e. negative (-) and positive (+) charge. The metal is usually positively charged while the nonmetal is usually negatively charged.
According to this question, the molecule beryllium chloride has one beryllium atom, a metal, and two chlorine atoms, nonmetals. This means that an ionic bond will be formed by beryllium atom and chlorine atom.
Learn more about ionic bonds at: https://brainly.com/question/11527546
#SPJ1
A solution is 5.00% by volume of ethanol dissolved in water. How many mL of ethanol are in 500 mL of the solution?
Answers
Answer:
\(v_{solute}=25mL\)
Explanation:
Hello there!
In this case, according to the by-volume concentration of this solution, it is possible for us to use its mathematical definition as shown below:
\(\%v=\frac{v_{solute}}{v_{solution}}*100\%\)
Thus, given the percent and the volume of the solution, we can solve for the volume of ethanol (solute) as shown below:
\(v_{solute}=\frac{\%v*v_{solution}}{100\% }\\\\v_{solute}=\frac{5.00\%*500mL}{100\%}\\\\ v_{solute}=25mL\)
Best regards!
Where are most volcanoes located? (Use information from the map.)
What is happening to the earth’s crust in these locations?
Answers
A new species of living thing is discovered. The cell theory states that which of the following must be true about
this new living thing?
A Its cells must be able to make their own food
All its cells must perform the same function
It must be made up of one or more cells
Some of its cells must include a cell wall.
Answers
The cell theory states that the new species must be made up of one or more cells.
Hence option (c) is correct.
According to the scientific theory known as "cell theory," which was initially put forward in the middle of the nineteenth century, all living beings are made up of cells, which also serve as the fundamental structural and organizing unit of all organisms.
All species are made up of cells, which are also the fundamental building block of reproduction.
The following three principles underlie the cell theory:
1) One or more cells make up every living thing.
2) The fundamental building block of structure and hierarchy in organisms is the cell.
3) From already existing cells, new cells form.
Hence according to the first principle, option (c) is correct.
Learn more about Cell theory here https://brainly.com/question/1594446
#SPJ9
What is the molecular formula of the compound with a molecular weight of 112 g/mol and percent composition: 85.6% C and 14.4% H?C8H16CH2C4H8C2H4
Answers
To find the molecular formula of this compound, what we're going to do is to follow up the steps:
Step 1: Pass all the percentages to grams. This is, just to change the unit:
Step 2: Divide each mass by respective molar mass to obtain the moles of each element:
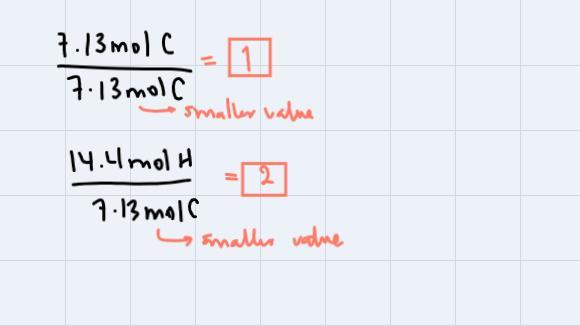
Step 3: Divide all the amounts in moles through by smaller value obtained:
These are the subscripts for C and H respectively. Thus our empirical formula is CH2. We're asked to find the molecular formula so, we could use the fact that the compound has a molecular weight of 112 g/mol. If we analyze, CH2 has a molecular weight of 14g/mol, so:
Step 4: We're going to divide the molecular weight of the compound with molecular formula through by the molecular weight of the compound with empirical formula:
This means that our molecular formula will be eight times the subscripts of the empirical formula. Therefore, the answer is:




EASY QUESTION PLEASE HELP I JUST NEED REASURANCE Is mass conserved when you fry an egg and it undergos a chemical change?
Answers
Answer:
Yeah chemical change occurs when you fry an egg since the whites harden and change shape in a way. Mass is conserved since it doesn't change.