Which statement is true because of Newton's second law?
When the net force on an object decreases, the object's acceleration increases.
When the net force on an object decreases, the acceleration doesn't change.
When the net force on an object increases, the object's acceleration decreases.
When the net force on an object decreases, the object's acceleration decreases.
Answers
Answer:
when the net force of an object decreases
, the objects acceleration decreases.
Answer:
D: When the net force on an object decreases, the object's acceleration decreases.
Explanation:
I took the quiz and got it right
Related Questions
Which causes an increase in greenhouse gases?
change in wind patterns
strengthening of hurricanes
burning of wood and coal
movement of warm ocean waters
Answers
Answer:
C
Explanation:
Took the test on egde
Burning of wood and coal causes an increases greenhouse gas.
What is greenhouse gas?A greenhouse gas absorbs as well as emits radiant energy in the thermal infrared region, resulting in the effect of greenhouse gas . Water vapor, carbon dioxide (\(CO_{2}\)), methane(\(CH_{4}\)), nitrous oxide, and ozone(\(O_{3}\)) are the principal greenhouse gases in the earth's atmosphere.
Generally, it is known that by burning of wood and coal carbon di-oxide gas will release. Therefore, burning of wood and coal will be responsible for greenhouse gas.
To know more about greenhouse gas.
https://brainly.com/question/4509458.
#SPJ2
Please help
How many moles of O2 are required to generate 12 moles SO2 gas? 2CuFeS2 + 502 → 2Cu + 2FeO + 4SO2 [ ? ] mol O₂ O2
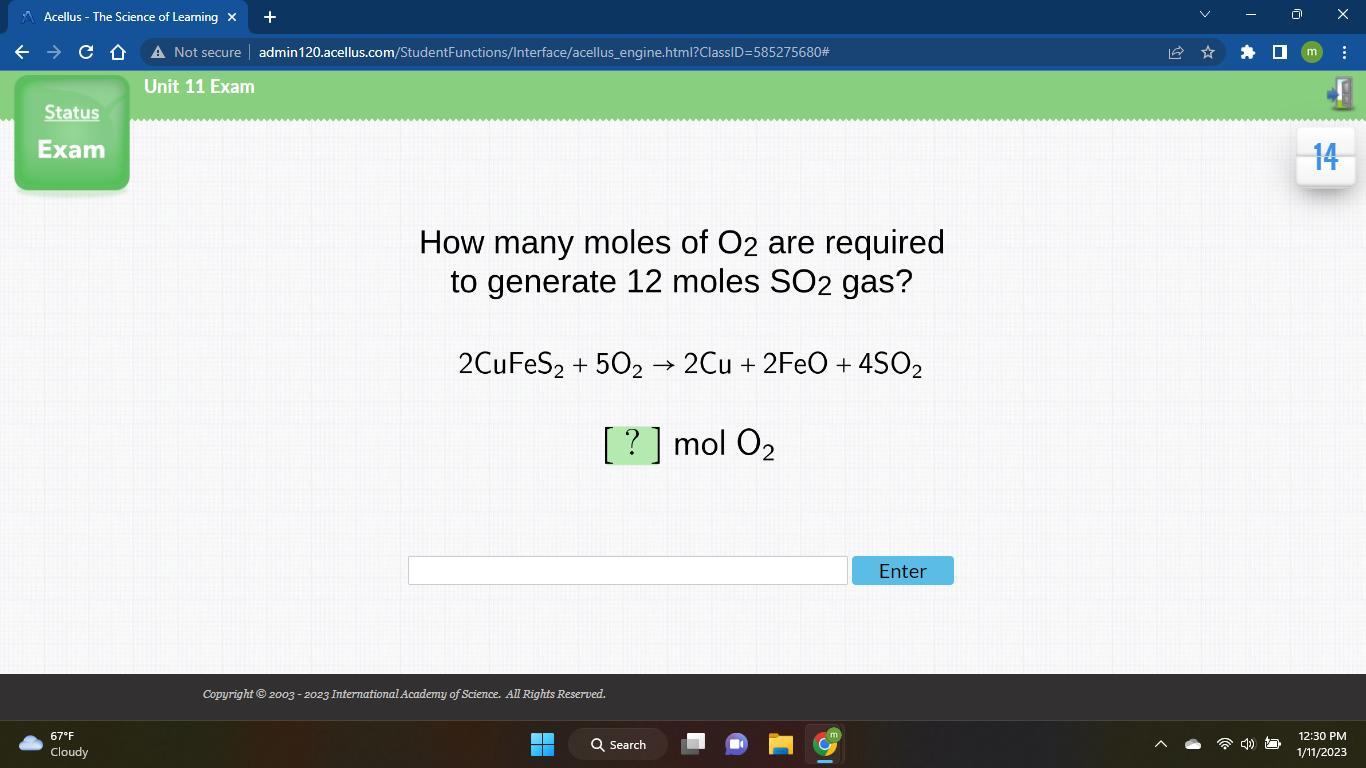
Answers
Answer:30 moles of oxygen are required if 12 moles of are consumed.
Explanation:The given balanced equation is:
From this balanced equation, there is 2:5 mol ratio between and .
We are asked to calculate the moles of required to react with 12 moles of .
It's a mol to mol conversion and the set up for this would be as:
=
So, 30 moles of oxygen are required to react with 12 moles of .
At a given temperature, 0.500 mols of CO and 1.50 moles of water vapor are added to a 2.50 L vessel. When the reaction reaches equilibrium, the [CO2] and [H2] are 0.00775 M. Find the [CO] and the [H2O] at equilibrium. Calculate the Keq and predict the sign of ΔG.
Answers
The concentrations of the reaction's reactants and products must be equal at equilibrium. Following is a description of how CO and H2O react to generate CO2 and H2: CO + H2O <=> CO2 + H2 We can determine the equilibrium CO and H2O concentrations using the available data.
The starting concentrations of CO and H2O are 0.800 M and 0.800 M, respectively, due to the total moles of CO and H2O being 2.00 moles and the total volume being 2.50 L. The equilibrium expression may be used to compute the equilibrium concentrations of CO and H2O: K = [CO2][H2]/[CO][H2O] K = (0.00775)(0.00775)/[CO] may be used to derive the equilibrium constant given that [CO2] and [H2] are both equal to 0.00775 M.
[H2O] K = (0.00775)(0.00775)/[0.0455], when the equilibrium concentrations of CO and H2O are plugged in.[0.0455]. ][0.0455] K = 0.0020 From this, we can calculate the equilibrium concentrations of CO and H2O: [CO] = 0.0455 M [H2O] = 0.0455 M .
The standard free energy change (G°), which can be calculated using the formula G° = -RTlnK, may be used to estimate the sign of G for this reaction. Since K > 1, we may anticipate a spontaneous response, meaning that G will be negative.
Learn more about concentrations at:
https://brainly.com/question/10725862
#SPJ1
simple chemical reactions and bonds
Answers
The process of changing one or more chemicals into new ones with distinct chemical characteristics is known as a chemical reaction.
Atoms share or exchange electrons in a chemical bond to achieve stability. Ionic, covalent, and metallic connections are the three basic forms of chemical bonds. One atom transfers an electron to another atom to create an ionic bond between two oppositely charged ions. When atoms share electrons to fill their outer energy level, covalent connections are created. Metals have metallic bonding because their electrons are not tightly bound to any one atom but rather move freely through a lattice of positive ions. Chemical or physical activities, such as heating or breaking, can both break bonds. When a molecule that dissociates bonds, like water, is added to a chemical reaction, bonds are broken. Bonds are broken in physical processes by exerting enough energy on them, such as during mechanical grinding or shearing. Bond energy or bond dissociation energy is the term used to describe the energy needed to dissolve bonds.
The complete question is:
Explain simple chemical reactions and bonds in detail, including the types of bonds and how they form and break?
Learn more about covalent here :
https://brainly.com/question/7357068
#SPJ4
How much heat is roguired to raise the temperature of 8.75 g of water from its melting point to its boiling pointsExpress your answer numerically in kilojoulos,
Answers
The heat required to raise the temperature of 8.75 g of water from its melting point to its boiling points is 3.662 kJ.
What exactly is specific heat?The amount of heat required to increase the temperature of one gram of a material by one degree Celsius (°C) is defined as specific heat.
What is the name of the specific heat formula?The equation q = mcΔt can be used to compute the amount of heat acquired or lost by a specific heat (q), where m is the mass of the sample, c is the specific heat, and Δt is the temperature change.
Given:
m = 8.75
c = 4.186 J/g°C
The melting point and boiling point of water is 0° and 100° respectively.
Δt = 100° - 0° = 100°
We know that,
q = mcΔt
= 8.75(4.186)100
= 3.662 kJ
Thus, the heat required to raise the temperature of 8.75 g of water from its melting point to its boiling points is 3.662 kJ.
Learn more about specific heat here:
https://brainly.com/question/21406849
#SPJ9
Generating a rate law is complicated when the rate-determining step is preceded by a?
Answers
By generating a rate law is complicated when the rate-determining step is preceded by 'a' is a Equilibrium reaction.
What is rate law?The rate law shows about the rate of chemical reaction depends on reactant concentration.
According to rate law, the rate of reaction is directly proportional to the concentration of reaction which is raised to a stiochiometric coefficient which is determined experimentally.
Thus, from above we concluded that if the preceding reaction is an equilibrium, that compromises the simplicity of writing the rate law from the stiochiometry of the rate determining step will be used up in the reverse reaction of the equilibrium.
learn more about rate law :
https://brainly.com/question/14779101
#SPJ4
Draw the exo and endo product for the reaction of cyclopentadiene and maleic anhydride. Which one will be favored?
Answers
The endo product is the favored product in the reaction between cyclopentadiene and maleic anhydride.When cyclopentadiene reacts with maleic anhydride, it undergoes a Diels-Alder reaction to form two different products, the exo and endo products.
The exo product is formed when the two substituents on the diene and dienophile are on the opposite sides of the newly formed ring. On the other hand, the endo product is formed when the two substituents are on the same side of the ring.
The endo product is typically favored in this reaction because it is more stable than the exo product. This is because the endo product has a more favorable overlap between the orbitals involved in the formation of the new sigma bond.
In conclusion, the Diels-Alder reaction between cyclopentadiene and maleic anhydride forms both exo and endo products, but the endo product is typically favored due to its greater stability.
Sub-heading: Drawing Exo and Endo Products
Step 1: Identify the reactants
- Cyclopentadiene: C5H6, a 5-membered ring with two adjacent double bonds.
- Maleic anhydride: C4H2O3, a cyclic molecule with an anhydride functional group.
Step 2: Determine the Diels-Alder reaction
- The reaction is a Diels-Alder reaction, which involves a conjugated diene (cyclopentadiene) reacting with a dienophile (maleic anhydride) to form a cyclic compound.
Step 3: Draw the exo product
- In the exo product, the two carbonyl oxygen atoms of maleic anhydride point away from the cyclopentadiene ring.
- To draw the exo product, connect one double bond of cyclopentadiene to one double bond of maleic anhydride, and the other double bond to the remaining double bond in maleic anhydride. Ensure the carbonyl oxygen atoms are pointing away from the cyclopentadiene ring.
Step 4: Draw the endo product
- In the endo product, the two carbonyl oxygen atoms of maleic anhydride point towards the cyclopentadiene ring.
- To draw the endo product, follow the same steps as for the exo product but make sure the carbonyl oxygen atoms are pointing towards the cyclopentadiene ring.
Favored Product
Step 5: Determine the favored product
- The endo product is favored in this reaction due to secondary orbital interactions that stabilize the transition state.
In conclusion, the endo product is the favored product in the reaction between cyclopentadiene and maleic anhydride.
To know more about Exo and Endo product refer to
https://brainly.com/question/29738690
#SPJ11
Below is a molecule of caffeine. Which of the following features are not present in a molecule of caffeine
Answers
Double bonds between carbon atoms: Caffeine contains several double bonds between carbon and nitrogen atoms, which contribute to its aromatic structure. These double bonds are not present in all molecules, but they are present in caffeine.
Chiral centers: Caffeine does not have any chiral centers. A chiral center is a carbon atom that is bonded to four different groups, resulting in non-superimposable mirror images. Caffeine lacks this structural arrangement, so it does not exhibit chirality.
Amino group: Caffeine does not contain an amino group (-NH2). Instead, it consists of three methyl groups (-CH3), two amide groups (-CONH), and several aromatic rings.
Sulfur atom: Caffeine does not contain any sulfur atoms. It is composed of carbon, hydrogen, nitrogen, and oxygen atoms.
Overall, caffeine is a complex molecule with unique features, including multiple aromatic rings and amide functional groups, but it does not possess double bonds between carbon atoms, chiral centers, an amino group, or a sulfur atom.
Learn more about carbon here;
brainly.com/question/13046593
#SPJ11
5. How much of the world’s land is used for agriculture?
A. 10 percent
B. 30 percent
C. 40 percent
D. 75 percent
23. How should all chemicals be stored?
A. In a clearly marked and approved container or cabinet
B. In a plastic container in the shop’s supply closet
C. On OSHA approved shelving
D. In a clear container
Answers
40 percent of the earth's land 11 percent of this is used only for crops and 1/4 is pastureland which is used for wild crops and grazing animals. The correct option is C
Chemicals should be stored in a clearly marked and approved container or cabinet. The correct option is A
What is agriculture?Agriculture can be defined as art and science of cultivating the soil, growing crops and raising livestock. It includes the preparation of plant and animal products for people to use and their distribution to markets
Therefore 40 percent of the earth's land 11 percent of this is used only for crops and 1/4 is pastureland which is used for wild crops and grazing animals.
Learn more Agriculture here: brainly.com/question/4755653
#SPJ1
Order the following events to describe how igneous rock can form at Mauna Loa

Answers
Answer:
3 then 4 then 5 then 1 then 2
Explanation:
ordering 1 through 5 from top to bottom
The correct order of igneous rock can form at Mauna Loa is as follows:
3Rock beneath Mauna Loa melts to form magma.
4 Melted rock rises toward Earth's surface.
5 Lava erupts from Mauna Loa and flows down the volcano.
1 Lava flowing down the volcano enters the Pacific Ocean.
2 Ocean water cools the lava, causing it to crystallize and form igneous rock.
What is an igneous rock ?Igneous rocks are the form when hot, molten rock crystallizes and solidifies. Igneous rock is produced through the cooling and solidification of magma or lava.
Igneous rocks produce when magma from inside the Earth moves toward the surface, or is forced above the Earth's surface as lava and ash by a volcano.
Thus, The correct order of igneous rock can form at Mauna Loa is 3, 4, 5, 1, and 2.
To learn more about the igneous rock, follow the link;
https://brainly.com/question/719105
#SPJ2
The specific heat capacity of liquid water is 4.18 J/g-K. How many joules of heat are needed to raise the temperature of 5.00 g of water from 15.0 °C to 36.5 °C?
Answers
The specific heat capacity of a substance is the amount of energy required to raise the temperature of 1g of the substance by 1K.
\(\begin{gathered} q=mc\Delta T \\ q:energy\text{ }(J)=x \\ m:mass\text{ }(g)=5.00g \\ c:specific\text{ }heat\text{ }capacity\text{ }(Jg^{-1}K^{-1}) \\ \Delta T:change\text{ }in\text{ }temperature\text{ }(K) \\ \Delta T:(final\text{ }temperature-initial\text{ }temperature) \end{gathered}\)Calculating the change in temperature:
\(\Delta T:(273.15K+36.5\degree C)-(273.15K+15\degree C)=21.5K\)By substituting what we are given into the equation to solve for the unknow x we have;
\(\begin{gathered} q=5.00g\times4.18Jg^{-1}K^{-1}\times21.5K \\ q=+449.35J \end{gathered}\)Answer: Energy needed is 449.35J
the molecular formula for vitamin c is c6h8o6. what is the empirical formula? question 2 options: cho ch2o c3h4o3 c2h4o2
Answers
c3h4c3
its the smallest number of atoms , so divide all values by 2 as that is the largest common factor of 6 and 8.
Use the sample data to construct a 90% confidence interval estimate of the percentage of cell phone users who develop cancer of the brain or nervous system. %
Answers
Using this formula, we can calculate the confidence interval once we have the sample data, without the sample data, it is not possible to provide an accurate confidence interval estimate.
To construct a 90% confidence interval estimate of the percentage of cell phone users who develop cancer of the brain or nervous system, we would need the sample data, specifically the number of cell phone users and the number of users who developed cancer. Without the sample data, it is not possible to provide an accurate confidence interval estimate.
However, if we assume that we have the necessary sample data, we can proceed with the calculation. The formula for calculating a confidence interval for a proportion is:
Confidence interval
\(=�^±�×�^(1−�^)�Confidence interval= p^ ±z× np^ (1− p^ ) where:�^p^\)
is the sample proportion (number of users with cancer divided by the total number of cell phone users).
\(�\)
z is the z-score corresponding to the desired confidence level (90% confidence level corresponds to a z-score of approximately 1.645).
\(�\)
n is the sample size (total number of cell phone users).
Using this formula, we can calculate the confidence interval once we have the sample data.
Learn more about confidence from below link
https://brainly.com/question/333719
#SPJ11
What are two possible contaminants and two possible pathways that containments can take to enter the water supply?
Fastest and correct gets brainliest!
Answers
Answer:
A contaminant can enter soil water, soil solids (mineral and organic phases), and soil air.
hat are the major products obtained upon treatment of ethyl methyl ether with excess HBr? Multiple Choice
1) Bromomethane and ethanol
2)Bromoethane and methanol
3)Bromoethane and bromomethane
4)Ethanol and methanat
Answers
Option 2) Bromoethane and methanol is correct
The major products obtained upon treatment of ethyl methyl ether with excess HBr are Bromoethane and methanol.
What is ethyl methyl ether?
Ethyl methyl ether is a colorless gas that is used as a solvent. The IUPAC name for this compound is methoxyethane. It is a member of the ether family of compounds. When ethyl methyl ether reacts with excess HBr, it undergoes a substitution reaction and forms Bromoethane and methanol. The mechanism for this reaction is given below: Methoxyethane reacts with hydrogen bromide to produce methanol and ethyl bromide (bromoethane). Here are the products that are formed in this reaction: Bromoethane (C2H5Br) and Methanol (CH3OH)
The chemical equation for this reaction can be written as: CH3OCH2CH3 + HBr → CH3OH + CH3CH2Br \(\boxed{Option\ 2)}\)
Learn more about ethyl methyl ether
https://brainly.com/question/31388194
#SPJ11
If the final mass of the system is 120g, what was the percent yield of the reaction?
Answers
Answer:
Sorry for the wrong answer plz
99% was the percent yield of the reaction. So, the correct option is A.
What is Percent yield?Percent yield is defined as the percentage ratio of the actual yield to the theoretical yield. It is a measure of the amount of moles of product formed in relation to the reactant obtained in a chemical reaction, expressed as a percentage.
For percent yield, we have to first find the number of moles.
1 Mg(S) + 2 HCl(Aq) → 1 MgCl2(Aq) + 1 H2(G)
Mole of Mg (s)= Mass of Mg/ Molar mass
=48/24= 2 moles
Moles of HCl= 73/36.5= 2moles
So, 1 mole of Mg reacts with 2 moles of HCl as per the equation which means HCl is the limiting reagent. Mg will act as excess reagent so only 1 mole of Mg will react while the unreacted Mg will be 24g i.e. 1 mole.
The product which is experimental= 120-24= 96g
Theoretical product formed= 1mole of \(MgCl_2\)+ 1 mole of HCl= 95+2= 97gm/mole
Percentage Yield = (experimental Yield / Theoretical Yield) × 100 %
= (96/97)*100 = 98.96% which is approx. 99%
Thus, 99% was the percent yield of the reaction. So, the correct option is A.
Learn more about Percent yield, here:
https://brainly.com/question/17042787
#SPJ2
Your question is incomplete, most probably the complete question is:
The Following Reaction Is Carried Out:
1 Mg(S) + 2 HCl(Aq) → 1 MgCl2(Aq) + 1 H2(G)
The Initial Masses Of The Uncombined Reactants appear below:
Mass of magnesium- 48g
mass of hydrochloric acid= 73g
The reactants are combined and the final mass of the system is determined. If the final mass of the system is 120g, what was the percent yield of the reaction?
99%50%1%25%in a redox-reaction that uses starch as an indicator, the solution turns blue-black. what can we say about that reaction?
Answers
Answer:
The reaction is nutride-cullic and sees all math about our lives.
Explanation:
- Why do we learn the Bohr Model?
Answers
Answer:
The Bohr model shows the atom as a small, positively charged nucleus surrounded by orbiting electrons. The Bohr model is simpler and relatively easy to understand. So,we learn the Bohr Model.
Phones are an essential mode of communication in today’s world. People use phones to communicate with their family members, colleagues, to call for help, and to educate others. Phones have transmitters and receivers within them because they both transmit and receive information. During this process, radio waves from a phone are transmitted to a cell tower. The tower receives the information and then transmits it to the phone of the person it is intended for. Remote areas often have poor cell phone reception because they do not have many cell towers nearby. Describe one way to solve this problem.
Answers
Answer:
Cell phones combine phone technology with computer technology, and the pairing has made an unprecedented amount of information available to humans all over the world.
What should Maria do before she turns on the
Bunsen burner to begin the experiment?
Place the classroom fire extinguisher next to
her lab bench.
Inspect the Bunsen burner and gas tubing for
damage.
Move her lab notebook and papers so they
are away from the burner.
Remove her safety goggles so she can see
the burner more clearly.
Answers
Answer:
For anyone that still needs the answer it's:
2.) Inspect the Bunsen burner and gas tubing for damage.
3.) Move her lab notebook and papers so they are away from the burner.
Explanation:
2 and 3 is right on edge
Bunsen burners are the laboratory equipment used in experiments. Maria should inspect the burner and move her papers and book away before burning. Thus, options b and c are correct.
What are bunsen burners?Bunsen burners are the equipment used in laboratories for heating and sterilizing processes. It is a device used to produce flames using atmospheric oxygen gas and fuel in the burner.
While using the bunsen burner, laboratory safety procedures should be practiced to ensure the safety of the person as well as the working place. Before igniting the burner should be inspected and checked for any leakages.
The notebooks and papers must be placed away from eh burner to avoid any fire accidents as they are easily flammable and can catch fire.
Therefore, options b and c. gas tubing must be inspected before lighting the burner.
Learn more about bunsen burner, here:
https://brainly.com/question/1477483
#SPJ6
Evaporation cools the liquid that is left behind because the molecules that leave the liquid during evaporation: A. have kinetic energy B.have greater than average speedeh C.Have broken the bonds that held them in the liquid. D. Create vapor pressure.
Answers
Create vapor pressure is behind because the molecules that leave the liquid during evaporation.
What is evaporation ?
The water cycle's crucial step is evaporation. When a liquid transforms into a gas, evaporation takes place. As rain puddles "disappear" on a hot day or when wet clothing dries in the sun, it is simple to envision. The liquid water in these instances is evaporating into a gas known as water vapor rather than actually dissipating.
What is kinetic energy ?
When an item undergoes work—the transfer of energy—by being subjected to a net force, it accelerates and acquires kinetic energy.
Therefore, Create vapor pressure is behind because the molecules that leave the liquid during evaporation.
Learn more about evaporation from the given link.
https://brainly.com/question/2013258
#SPJ1
In your own words, explain Earth's atmosphere.
Answers
Answer:
yes
Explanation:
because the atmosphere is the shield of earth
If you initially have a gas with a pressure of 0.950 atm and a temperature of 35 °C and you heat it to 65 °C, what is the new pressure if volume and moles are held constant? (P2 = 1.04 atm)
Answers
Explanation:
fgIf the piston moves so as to increase the pressure of the gas to 2.94 atm, ... new volume of the cylinder? ... A gas has a pressure of 699.0 mm Hg at 40.0°C. What is the temperature (in C) ...
Which parts of atoms can interact (react) to form chemical bonds? valence electrons protons the nucleus of each atom the orbitals
Answers
The valence electrons are the components of atoms that can interact or react to form chemical bonds.
These are the electrons located in the outermost energy level or shell of an atom.
They participate in chemical bonding and decide an element's reactivity and chemical characteristics.
Each atom's nucleus and protons are not directly involved in the formation of chemical bonds. On the other side, orbitals are areas of an atom where electrons are most likely to be present, but they don't interact or react with one another to create chemical bonds.
To know more about valence electrons click on below link :
https://brainly.com/question/31264554#
#SPJ11
In an atom that has lost an electron, the place vacated by the free electron is referred to as a(n)
Answers
The space left by the free electron in an atom after it has lost one electron is known as a hole.
The term "free electron" refers to an electron that has broken loose from its "parent" atom and is now free to move about the material at random. A vacancy, also known as a hole, is created in the atom when a bonded electron in a lattice atom breaks free. The number of electrons in an atom's valence shell, or n shell in this example, has a significant impact on the electrical properties of the element that the atom represents. The least force of attraction is felt by the electrons in the valence shell since they are the furthest from their nucleus.
To learn more about atom click here https://brainly.com/question/1566330
#SPJ4
I NEED HELP PLEASE THANK YOUUU

Answers
I think the answer is c if not then try a
Answer: B
Explanation:
How is a polar compound formed?
Answers
Answer: when two atoms do not share electrons equally in a covalent bond.
Explanation:
1. You are given the number of moles of carbon and must convert it to an equivalent mass using the molar mass from the periodic table. The carbon sample is 0.045 moles.
2. How many moles of potassium are in 525.0 g of pure potassium? Explain
Answers
0.54g is the mass of carbon in 0.045 moles of carbon. Elementary particles shared the same quantity of matter.
What is mass?A body's mass is an inherent attribute. Until the discoveries of the atom as well as particle physics, it was thought to be tied to the amount of matter inside a physical body. It was discovered that various atoms and elementary particles shared the same quantity of matter.
mole = given mass/ molar mass
substituting all the given values in the above equation, we get
0.045 moles = mass/ 12
mass =0.045×12= 0.54g
Therefore, 0.54g is the mass of carbon in 0.045 moles of carbon.
To learn more about mass, here:
https://brainly.com/question/15368078
#SPJ1
A solution is made by adding 60 g table salt to 100 mL water. The solubility of salt is 36 g/100 mL water.
Which term best describes this solution?
dilute
saturated
supersaturated- The Answer
unsaturated
Answers
Answer:its c
Explanation:
Answer: unsaturated
Explanation: because i just took the test
How many grams are in 1.3 moles of Cr?
Answers
Answer:
40.38 grams
Explanation: