Which statements explain why theories change over time? Check all that apply.
Scientists change the definition of theory to have their ideas accepted.
New information and technology may be developed that influence the theory.
Theories change with each new generation of scientists.
New experimental methods provide new information.
Theories may or may not be supported by new information.
Answers
The following are true about a theory;
New information and technology may be developed that influence the theory.New experimental methods provide new information.Theories may or may not be supported by new information.What is a theory?A theory is an explanation that is given to a phenomenon which may have some kind of underpinning reasoning that may not necessarily be empirical. Thus a theory has withstood some kind of scrutiny but is not yet absolutely accepted as true.
Thus the following are true about theory;
New information and technology may be developed that influence the theory.New experimental methods provide new information.Theories may or may not be supported by new information.Learn more about theory:https://brainly.com/question/1759635
#SPJ1
Related Questions
The image shows a representative sample of 50
atoms of a fictious element, called lemonium ( Le ). Lemonium consists of three isotopes. The red spheres are Le– 19 , the light blue spheres are Le– 17 , and the dark blue spheres are Le– 15. Determine the percent natural abundance of each of the three isotopes.
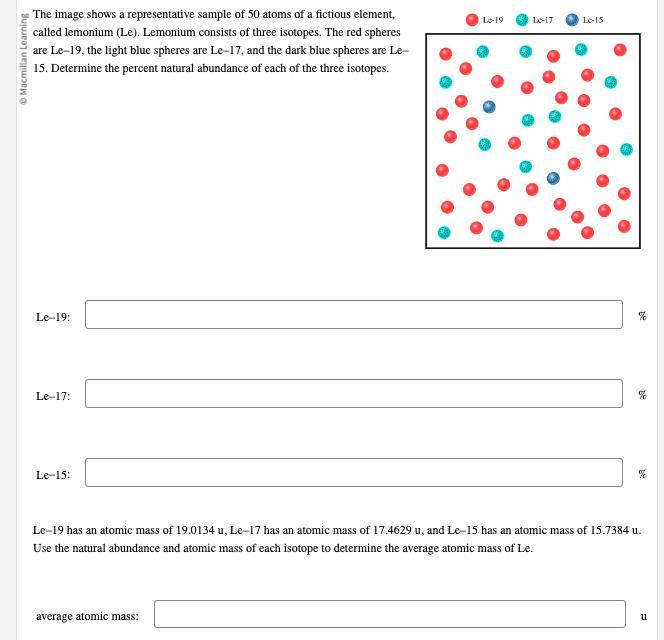
Answers
The percent natural abundance of Le-19, Le-17, and Le-15 isotopes are 40%, 44%, and 16% respectively.
Lemonium (Le) has three isotopes, which are Le-19, Le-17, and Le-15.
The given image shows a representative sample of 50 atoms of Lemonium, and we are to determine the percent natural abundance of each of the three isotopes.
Lemonium is an element having three isotopes, so we need to calculate the percent natural abundance of each of the three isotopes of Lemonium.
The percent natural abundance of the isotopes can be calculated as follows:Percent natural abundance of Le-19:As we know that Lemonium (Le) has three isotopes, so it can be represented as follows: Le-19, Le-17, and Le-15.
We are given that the number of Le-19 isotopes in the representative sample is 20.
So, the percentage of Le-19 isotopes can be calculated as follows:Percentage of Le-19 = (Number of Le-19 isotopes / Total number of Lemonium atoms) x 100% = (20/50) x 100% = 40%.
Therefore, the percent natural abundance of Le-19 is 40%.
Percent natural abundance of Le-17:Similarly, the number of Le-17 isotopes in the representative sample is 22.
So, the percentage of Le-17 isotopes can be calculated as follows:Percentage of Le-17 = (Number of Le-17 isotopes / Total number of Lemonium atoms) x 100% = (22/50) x 100% = 44%.
Therefore, the percent natural abundance of Le-17 is 44%.
Percent natural abundance of Le-15:Moreover, the number of Le-15 isotopes in the representative sample is 8.
So, the percentage of Le-15 isotopes can be calculated as follows:Percentage of Le-15 = (Number of Le-15 isotopes / Total number of Lemonium atoms) x 100% = (8/50) x 100% = 16%.
Therefore, the percent natural abundance of Le-15 is 16%.
For more such questions on isotopes
https://brainly.com/question/14220416
#SPJ8
Write the name and the symbol of the element that satisfies the following conditions:

Answers
Answer:
Sulphur(S).......Answer:
Argon Ar is the element that satisfies the following condition
Students want to gather evidence for the claim that the number of atoms present before a chemical reaction is equal to the number of atoms present after the chemical reaction. They decide to react vinegar and baking soda in a sealed plastic bag. Which of the following will provide the evidence the students need?
A. The mass of the plastic bag, baking soda, and vinegar before the reaction was equal to the mass after the reaction.
B. Bubbles were produced during the reaction, which meant that a gas was being produced.
C. The plastic bag did not change in any way, indicating that it was not involved in the reaction.
D. The mass of the baking soda was exactly equal to the mass of the vinegar used to create the chemical reaction. THIS IS DUE IN AN HOUR HELPPP!!!
Answers
The mass of the plastic bag, baking soda, and vinegar before the reaction was equal to the mass after the reaction. Option A.
Law of conservation of atomsAtoms, mass, and energy are conserved during chemical reactions. Even though their forms may change after reactions.
For a reaction involving vinegar and baking soda, the equation can be expressed as:
vinegar + baking soda ---> carbon dioxide + sodium acetate + water
or
\(CH_3COOH (aq) + NaHCO_3 (aq) --- > CH_3COONa (aq) + H_2O (l) + CO_2 (g)\)
In other words, sodium acetate, water, and carbon dioxide gas are produced in the reaction. Assuming that the reaction vessel is not sealed, the gas would escape away from the vessel.
Since the vessel was sealed, the mass of the plastic bag, baking soda, and vinegar before the reaction will be equal to the sum of the masses of the plastic bag and the products after the reaction.
This will show that the number of atoms present before the reaction is the same as the number of atoms present after the reaction.
More on the law of conservation of atoms can be found here: https://brainly.com/question/20635180
#SPJ1
The continental crust is made of?
Answers
Answer:
It is made out of granite.
Explanation:
This is because of the tectonic plates.

how many moles of CO2 are formed from 3.0 mol of C2H2
Answers
Answer:
50.0 moles C02
Explanation:
First write down the CORRECTLY balanced equation. NOTE: The equation you provide is incorrect.
2C2H2(g) + 5O2(g) ==> 4CO2(g) + 2H2O(g) CORRECT EQUATION
Next, look at the stoichiometric ratio of C2H2 to CO2. You can see it is 2 moles C2H2 produces 4 moles CO2.
Thus, 25.0 moles C2H2 x 4 moles CO2/2 moles C2H4 = 50.0 moles CO2
Consider two gaseous systems: one for which the Vrms of the particles is low and another for
which the Vrms of the particles is high. What can you say about the relative temperatures of
each system?
Answers
Answer:
See explanation
Explanation:
According to chemistry libretexts(2020); "The rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. ... As the temperature of a gas is increased, the velocity of the molecules is also increased."
Hence considering two gases for which the Vrms of the particles of one is high and that of the other is low, we can conclude that the gas having the higher Vrms is at a higher temperature than the gas having a lower Vrms according to the foregoing.
If a dilute solution of hydrochloric acid is electrolysed, what gas will be produced at the anode? Answer in words not symbols.
Answers
If a dilute solution of hydrochloric acid (HCl) is electrolyzed, the gas produced at the anode (positive electrode) will be chlorine gas (Cl₂). When a dilute solution of hydrochloric acid (HCl) is electrolyzed, it undergoes a process called electrolysis.
In the case of hydrochloric acid, it dissociates into hydrogen ions (H+) and chloride ions (Cl-). The positive hydrogen ions (H+) are attracted to the cathode (negative electrode) and are involved in the reduction reaction. At the cathode, hydrogen gas (H₂) is produced as a result of the reduction of H+ ions. At the anode (positive electrode), the chloride ions (Cl-) are attracted. Here, the chloride ions undergo oxidation, losing electrons and forming chlorine gas (Cl₂). The chlorine gas is released as a product of the reaction at the anode.
Learn more about the gas here
https://brainly.com/question/28392063
#SPJ1
Which of the following are made possible by energy transfer from the Sun? Choose all that apply.
A. biofuels
B. nuclear power using uranium
C. wind power
D. hydroelectric power using a dam
This is Really Science but I couldn’t find it but please help ASAP!
Answers
Which is the order of these solutions from strong acid to strong base?
Solutions:
household ammonia
battery acid
baking soda
stomach acid
antacid
A-stomach acid household ammonia battery acid antacid baking soda
B-battery acid stomach acid antacid baking soda household ammonia
C-battery acid stomach acid baking soda antacid household ammonia
D-stomach acid battery acid antacid baking soda household ammonia
Answers
Answer:
First is battery acid with a pH of 1, second is stomach acid with a pH of 1.5-3.5 (depends), third is antacid with a pH of 7, fourth is baking soda with a pH of 9, and finally, fifth is household ammonia with a pH of 11.
Here is a picture of the pH levels to better help you understand.

Answer:
b
Explanation:
Mg(s) + Ni2+(ag) -> Mg2+ (aq) + Ni(s) What is the total number of moles of electrons lost by Mg(s) when 2.0 moles of electrons are gained by Ni2+(ag)? * 10 ( 1.0 mol ,20 mol ,3.0 mol, 4.0 mol
Answers
The total number of moles of electrons lost by Mg(s) when 2.0 moles of electrons are gained by Ni2+(ag) is also 2.0 moles of electrons.
How to find the number of moles?This is because in a chemical equation, the number of moles of electrons gained by the reducing agent (in this case Ni2+) is equal to the number of moles of electrons lost by the oxidizing agent (in this case Mg(s)).
In this redox reaction, Mg is being oxidized because it loses electrons and Ni is being reduced because it gains electrons. The oxidation and reduction process are occurring simultaneously, so the number of electrons lost by Mg(s) is equal to the number of electrons gained by Ni2+(ag).
Learn more about moles of electrons in brainly.com/question/512038
#SPJ1
The electrons that are gained by the \(Ni^{2+}\) ion is 2.0 moles of electrons.
What is the number of the electrons gained?We know that when there is a redox reaction, there would be the loss or gain of electrons in the process. The process is a simultaneous one so the electrons that are lost by one specie must as a matter of necessity be gained by another specie.
In this case, as we look at the reaction equation we can see that there are two electrons that have been lost by the magnesium atom and these two electrons would be gained by the Nickel II ion.
Learn more about redox reaction:https://brainly.com/question/13293425
#SPJ1
What type of reaction is shown below?
2N₂ + 3H₂ <==>2NH3
A. A reversible reaction
B. A combustion reaction
C. A one way reaction

Answers
The given chemical equation, 2N₂ + 3H₂ <==>2NH3, is an example of a reversible reaction.option A.
The double arrows between the reactants and products indicate that the reaction can proceed in both directions, forming both products and reactants.The reversible reaction, also known as a chemical equilibrium reaction, refers to a chemical reaction that can occur in both forward and reverse directions. It occurs when reactants are converted into products, and the products are also converted back into the original reactants.A reversible reaction can be identified by the symbol “<==>” or “⇌” that appears between the reactants and products in the chemical equation. It denotes that the reaction is in a state of chemical equilibrium. When the reactants and products have achieved equilibrium, the rate of the forward and reverse reactions is equal, and there is no further net change in the amounts of the reactants and products.A combustion reaction is a type of exothermic reaction in which a substance reacts with an oxidizing agent to produce heat and light. A one-way reaction is a type of reaction that occurs in only one direction and cannot be reversed without significant changes to the reaction conditions, such as changing the temperature or pressure.A reversible reaction, unlike a one-way reaction, can occur in both directions and reach equilibrium when the forward and reverse reaction rates are equal.option A.
for such more questions on reaction
https://brainly.com/question/24795637
#SPJ8
A chemist has 2.0 mol of methanol (CH3OH). The molar mass of methanol is 32.0 g/mol. What is the mass, in grams, of the sample? 16 30 32 64
Answers
Answer:
\(\boxed {\boxed {\sf D. \ 64 \ grams }}\)
Explanation:
Given the moles, we are asked to find the mass of a sample.
We know that the molar mass of methanol is 32.0 grams per mole. We can use this number as a fraction or ratio.
\(\frac{32 \ g \ CH_3OH}{1 \ mol \ CH_3OH}\)
Multiply by the given number of moles, which is 2.0
\(2.0 \ mol \ CH_3OH *\frac{32 \ g \ CH_3OH}{1 \ mol \ CH_3OH}\)
The moles of methanol will cancel each other out.
\(2.0 \ *\frac{32 \ g \ CH_3OH}{1 }\)
The denominator of 1 can be ignored.
\(2.0 * 32 \ g\ CH_3OH\)
Multiply.
\(64 \ g \ CH_3OH\)
There are 64 grams of methanol in the sample.
The answer is 64 grams, or:
D. 64Consider the following reversible reaction. Upper C upper O (g) plus 2 upper H subscript 2 (g) double-headed arrow upper C upper H subscript 3 upper O upper H (g). What is the equilibrium constant expression for the given system? K e q equals StartFraction StartBracket upper C upper O EndBracket StartBracket upper H 2 EndBracket superscript 2 over StartBracket upper C upper H subscript 3 upper O upper H EndBracket EndFraction. K e q equals StartFraction StartBracket upper C upper H subscript 3 upper H upper O EndBracket over StartBracket upper C upper O EndBracket StartBracket upper H 2 EndBracket superscript 2 EndFraction. K e q equals StartFraction StartBracket upper C upper O EndBracket StartBracket upper H 2 EndBracket over StartBracket upper C upper H subscript 3 upper O upper H EndBracket EndFraction. K e q equals sart fraction StartBracket upper C upper H subscript 3 upper O StartBracket upper C upper O EndBracket StartBracket upper H 2 EndBracket EndFraction.
Answers
Answer:
\(Keq=\frac{[CH_3OH]}{[CO][H_2]^2}\)
Explanation:
Hello,
In this case, for the described reaction at equilibrium:
\(CO(g)+2H_2(g)\rightleftharpoons CH_3OH(g)\)
The analysis of the law of mass action allows us to write the equilibrium expression as shown below:
\(Keq=\frac{[CH_3OH]}{[CO][H_2]^2}\)
Which is written considering that carbon monoxide, hydrogen and methanol are all in gaseous phase, for that reason all of them are included in the expression due to homogeneous equilibrium. Moreover, since hydrogen has a stoichiometric coefficient of 2, it is squared in the law of mass action.
Best regards.
Answer:
the answer is B if your looking for the letter
Explanation:
K e q equals StartFraction StartBracket upper C upper H subscript 3 upper H upper O EndBracket over StartBracket upper C upper O EndBracket StartBracket upper H 2 EndBracket superscript 2 EndFraction.
4 Al(s) + 3 O2(g) > 2 Al2O3(s)How many grams of Al2O3 can be formed from 32.19 g of Al and 50.22 g of O2?(Hint: You need to determine which one is the limiting reactant).
Answers
1) Chemical equation
\(4Al_{(s)}+3O_{2(g)}\rightarrow2Al_2O_{3(s)}\)2) Convert grams to moles
Al: 32.19g
The molar mass of Al is 26.98 g/mol
\(molAl=32.19gAl\cdot\frac{1molAl_{}}{26.98gAl_{}}=_{}1.19molAl_{}\)We have 1.19 mol Al.
O2: 50.22g
The molar mass of O2 is 31.999 g/mol
\(molO_2=50.22gO_2\cdot\frac{1molO_2}{31.999gO_2}=1.57molO_2\)We have 1.57 mol O2.
3) Limiting reactant
How many moles of O2 do we need to use all of the Al?
The molar ratio between O2 and Al is 3 mol O2: 4 mol Al.
\(molO_2=1.19molAl\cdot\frac{3molO_2}{4molAl_{}}_{}=0.8925molO_2\)We need 0.8925 mol O2 and we have 1.57 mol O2. We have enough O2.
O2 is the excess reactant.
How many moles of Al do we need to use all of the O2?
The molar ratio between O2 and Al is 3 mol O2: 4 mol Al.
\(molAl=1.57molO_2\cdot\frac{4molAl_{}}{3molO_2}=2.09molAl_{}\)We need 2.09 mol Al and we have 1.19 mol Al. We do not have enough Al.
Al is the limiting reactant.
4) Moles of Al2O3 produced from 1.19 mol Al.
The molar ratio between Al and Al2O3 is 4 mol Al: 2 mol Al2O3.
\(mol_{}Al_2O_3=1.19molAl\cdot\frac{2molAl_2O_3}{4molAl}_{}=0.595molAl_2O_3\)5) Convert moles to grams
The molar mass of Al2O3 is 101.96 g/mol
\(gAl_2O_3=0.595molAl_2O_3\cdot\frac{101.96gAl_2O_3}{1molAl_2O_3}=60.67gAl_2O_3\)The mass of Al2O3 produced is 60.67 g.
How do computers play a role in meteorology? Select three options.
They use equations to predict future weather.
They collect data from weather stations.
They draw weather maps.
They measure temperature, humidity, and wind speed.
They send out microwaves to make radar measurements.
Answers
Answer:
a b c :]
Explanation:
Computers play a role in meteorology by equating to;
predict future weather,collect data and draw weather maps etc.Computers in MeteorologyThere options that best describe computers in Meteorology above are:
They use equations to predict future weather.They collect data from weather stations.They draw weather maps.Read more on Meteorology:
https://brainly.com/question/11857753
#SPJ2
Non Polar covalent bonds are between
Answers
Answer:
atoms that share a pair of electrons with each other
Explanation:
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
Convert 9.13 decigram into milligram
Answers
Answer:
(9.13)
multiply the mass value by 100
=913 milligrams
Answer:
913 mg
Explanation:
913
URGENT!!!
5. As the water temperature is increased
from 20°C to 90°C, how many more grams
of copper sulfate will dissolve in 100 grams
of water?
____g
Answers
Answer:
40
Explanation:
what can chemically be broken down into a simpler substance
Answers
Answer: ea sports
Explanation:
A compound is a substance that contains two or more elements chemically combined in a fixed proportion. However, it is not an element because it can be broken down into simpler substances – carbon and hydrogen. ... Recall that the components of a mixture can be separated from one another by physical means.
the phenol used in some mouthwashes and throat lozenges is . selected answer: correctc. hexylresorcinol answers: a. cannabinol b. cresol correctc. hexylresorcinol d. hexachlorophene
Answers
The correct answer to the question about the phenol used in some mouthwashes and throat lozenges is option C, hexylresorcinol.
This compound is a type of phenol that is used as an antiseptic and disinfectant in various products, including mouthwashes and throat lozenges.
It is effective in killing bacteria and other microorganisms that can cause infections and other health problems.
Therefore, the correct answer to this question is hexylresorcinol.
Hexylresorcinol is a substituted phenol with bactericidal, antihelminthic and potential antineoplastic activities.
Hexylresorcinol is used as an antiseptic in mouthwashes and skin wound cleansers.
The antibacterial effects of hexylresorcinol in vivo may be mediated through several mechanisms including reducing bacterial adherence to the pharynx, inhibiting bacterial biofilm formation, disrupting bacterial cell chain formation, and modifying cell surface hydrophobicity
To know more about phenol, refer here:
https://brainly.com/question/30650792#
#SPJ11
Identify the chemical reactions as endothermic, exothermic, or neither

Answers
Answer:
egg: endothermic
candle: exothermic
plaster and water : exothermic
salt and water: neither
Answer:
he is right
Explanation:
Please help (20 points)

Answers
Answer:
universe, galaxy, solar system, planet
The irreversible elementary gas-phase reaction is carried out isothermally at 305 K in a packed-bed reactor with 100 kg of catalyst. The entering pressure was 20 atm and the exit pressure is 2 atm. The feed is equal molar in A and B and the flow is in the turbulent flow regime, with F A0 10 mol/min and C A0 0.4 mol/dm 3 . Currently 80% conversion is achieved. What would be the conversion if the catalyst particle size were doubled and everything else remained the same
Answers
Answer:
0.856
Explanation:
Given data:
Feed molar rate ( Fao ) = 10 mol/min
Feed concentration ( Cao ) = 0.4 mol/dm^3
current conversion rate = 80%
Temperature = 305 K
catalyst = 100 kg
entering pressure = 20 atm
exit pressure = 2 atm
Determine the conversion if the catalyst particle size were doubled and everything else remained the same
The conversion if the catalyst particle size were doubled and other factors remains the same = 0.856
attached below is a detailed solution


What observations and reasoning led to the development of Hubble's Law?
Answers
Answer:
Hubble's law says that the universe is expanding outward.
Explanation:
Actually Hubble's law was discovered before the Big Bang theory was formulated. The Big Bang Theory is an attempt to explain the observations that led to Hubble's Law.Before the 1900s the theory was that the universe was eternal and self existent. The idea was that the universe was in a steady state having always existed and would always continue to exist. Albert Einstein even changed the equations in his general theory of relativity to reflect the idea of a steady state. Later he called putting in a fudge factor to result in a steady state the worse mistake of his life.
Hubble observed that most of the universe has a red shift indicating that the universe is expanding and moving away from itself. The further out that the universe is observed the faster it is moving apart.
These observations were inconsistent with a steady state universe.
The Big Bang theory extrapolated backwards. If the universe is expanding and spreading out from itself then further back in time the universe was closer together. The Theory explained Hubble's observations by the idea that at the beginning of time ( for our universe) all the matter and energy were together in one place.
This super dense ball of matter and energy then exploded outwards creating space and time as it is presently observed. The question was would the forces of gravity and black holes bring the matter and energy back together again. The answer found in 1998 was no. The rate of the expansion of the universe is increasing not slowing down and the universe will not collapse back into the super dense ball of matter that it began as.
The Big Bang Theory postulated based on the empirical evidence that our universe had a beginning and it will eventually cease to exist. The conclusion based on Hubble's observations is that matter and energy are not eternal and self existent.
3. If an element has 47 protons and 54 neutrons what is the element and what is its atomic
mass?
4. If one atom has 47 neutrons and a mass of 87 and another one has 41 protons and a
mass of 87, are they isotopes of each other?
5. Draw the electron dot diagram for the element Phosphorous.
Answers
4.Nb(Niobium) the atomic mass is 92.906
5.

The median of the following set of data (39.8, 39.6, 39.2, 39.6 and 39.5) is:
Answers
The median of the following set of data (39.8, 39.6, 39.2, 39.6 and 39.5) is 39.6.
What is median?Median is a number separating the higher half from the lower half of a data sample, population, or probability distribution.
The median of a finite list of numbers can be found by arranging all the observations from lowest value to highest value and picking the middle one (e.g., the median of {3, 3, 5, 9, 11} is 5.
The following set of data was given this question;
39.8, 39.6, 39.2, 39.6 and 39.5
We arrange these data as follows:
39.2, 39.5, 39.6, 39.6, 39.8
The middle number or median is 39.6
Learn more about median at: https://brainly.com/question/28060453
#SPJ1
Polyester and Nylon are examples of synthetic materials called polymers.
A. True
B. False
Answers
Answer:
true is correct
Polyester and Nylon are examples of synthetic materials called polymers. This statement is True.
Polymers are large molecules composed of repeating subunits called monomers, which are chemically bonded together. In the case of polyester, it is made from a polymer called polyethylene terephthalate (PET), while Nylon is made from polyamide monomers. These polymers are created through polymerization processes that combine smaller molecules to form long chains.
Synthetic polymers like polyester and nylon are widely used in various applications due to their desirable properties, such as strength, durability, and resistance to chemicals and moisture.
Learn more about Polymers, here:
https://brainly.com/question/8930717
#SPJ3
Which side of this proton transfer reaction is favored at equilibrium?

Answers
The left side proton transfer will be favored and the reactants will convert into products , Option C is the right answer.
What is Proton Transfer ?A step in a process in which the proton is removed from one species and accepted by another species is called Proton Transfer.
It generally takes place between an acid and a base.
At equilibrium the reactants are mostly converted into products , At equilibrium the equilibrium lie towards the right side .
Therefore left side proton transfer will be favored and the reactants will convert into products.
To know more about Proton Transfer
https://brainly.com/question/861100
#SPJ1
Solder is a silver metal used to hold pipes together. When the solder is heated, it melts and acts as a type of metal "glue." Mrs. Hanley heats a piece of solder until it melts between two pipes. What best identifies the point at which a physical change first takes place?
A)when the solder melts
B)when the solder cools down
C)when the solder is a soft metal
D)when the solder becomes solid again
Answers
Answer:
A
Explanation: