Which substances are included in the equilibrium constant expression, kc?.
Answers
Equilibrium constant expression (Kc) is an expression that shows the relationship between the reactants and products of a chemical reaction at equilibrium, at a specific temperature and pressure. Kc is defined as the ratio of the product concentration raised to their stoichiometric coefficients to reactant concentrations raised to their stoichiometric coefficients.
The substances included in the equilibrium constant expression, Kc are those which are involved in the chemical reaction that has reached equilibrium. In other words, Kc only includes the concentration of reactants and products that are present at equilibrium.
Therefore, only substances that are in the reaction equation are included in the Kc expression. The expression Kc is only valid for reactions in a homogeneous phase, which means all the reactants and products must be in the same phase, either gas or liquid.
To know more about constant visit:
https://brainly.com/question/31730278
#SPJ11
Related Questions
What is a stable electron configuration?
Answers
12. MnO4 + A. B. From the equation below, which is oxidizing agent Mn²+ C. D. SO₂ - MnO4 SO₂ Mn²+ 2 SO4² + SO
Answers
MnO4- acts as an oxidizing agent, whereas SO2 acts as a reducing agent.
What is oxidising agent?An oxidizing agent is a chemical species that causes oxidation in another substance by receiving electrons from it. To put it another way, it is a chemical that makes it easier for electrons to move from the object being oxidized to itself.
When an oxidation occurs, electrons are lost or the oxidation state is increased; when a reduction occurs, electrons are gained or the oxidation state is decreased.
In this equation, MnO4- + SO2 + H2O → Mn2+ + SO42- + 2H+
Because it causes SO2 to undergo oxidation (i.e., lose electrons) and goes through reduction itself, MnO4- is the oxidizing agent in this equation (i.e., gains electrons).
Due of its ability to both reduce MnO4- and oxidize itself, SO2 is the reducing agent.
Mn2+ is not an oxidizing agent because it is the end result of the reduction of MnO4-.
As SO42- is a byproduct of SO2 oxidation, it cannot act as a reducing agent.
MnO4- is therefore the oxidizing agent, whereas SO2 is the reducing agent.
To know more about oxidising agent, visit:
https://brainly.com/question/30281967
#SPJ1
Dna has an important role in making proteins, the biomolecules that help determine our traits as well as take part in chemical reactions throughout our bodies. Can you correctly label the steps/parts involved in protein synthesis?.
Answers
Protein synthesis is a process that is generated within cells where different organelles and genetic components are involved through which proteins are generated.
What are the steps of protein formation?The biosynthesis of proteins will be given by different parts, beginning with the translation of the genetic material. It will be given by different steps:
1. mRNA binds to ribosomes and the aminoacyl-tRNA to be associated to the first codon of the mRNA is associated.
2. The elongation of the polypeptide chain occurs, in which the amino acids are joined
3. The elongation is finished when the termination codons are reached.
4. The translation components are released and post-translational modifications are started.
5. The folding, glycosylation and modification of amino acids occur
6. Finally, the protein is released to generate its function
To learn more about protein synthesis visit: https://brainly.com/question/29765585
#SPJ1
checmical reactions that take place in the body are mediated by:
Answers
Chemical reactions that take place in the body are mediated by enzymes. Enzymes are proteins that act as catalysts in chemical reactions, speeding up the process without being consumed or changed in the reaction.
Enzymes are proteins that act as catalysts in chemical reactions. They work by forming an active site on their surface that binds to reactants in the reaction, then lowering the activation energy required to form the products. This means that they speed up the process without being consumed or changed in the reaction.
Enzymes are basically the proteins that help speed up metabolism, or the chemical reactions in human bodies. They usually build some substances and break the others down.
All living things have enzymes in them. Our bodies naturally produce these enzymes. But enzymes are also present in manufactured products and food.
To know more about chemical reaction refer to-
brainly.com/question/29039149#
#SPJ11
What structural feature in a molecule is necessary for a gas to absorb ir radiation?.
Answers
The ability to absorb infrared radiation is a feature of some atmospheric gases. The two main gases in the atmosphere, oxygen and nitrogen, lack this feature. It is known as the absorption of IR energy when infrared light impacts a molecule, like carbon dioxide, and causes the bonds to flex and vibrate.
Molecular motions must alter the molecule's dipole moment in order for them to absorb infrared radiation. This requirement is met by all molecules having three or more atoms, making them all IR absorbers.
Molecular entities with a modest energy gap between the vibrational and rotational states commonly absorb IR light. A net change in a molecule's dipole moment as it oscillates or spins is a requirement for IR absorption.
Infrared (IR) energy can be absorbed by carbon dioxide (CO2) molecule.
The infrared radiation released by the Earth's surface is trapped by greenhouse gases such water vapour, carbon dioxide, methane, and nitrous oxide.
To know more about I R Radiation, click on the link below:
https://brainly.com/question/14053556
#SPJ4
I need help answering these

Answers
U has a total of six electrons. This corresponds to carbon (C). A is the second most common element in the atmosphere.
How to explain the informationThe second most common element in the atmosphere is oxygen (O). E is a noble gas.
Noble gases include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Based on the given options, E could be xenon (Xe).
S is an alkali metal.
Alkali metals include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). Based on the given options, S could be sodium (Na).
O is a halogen.
Halogens include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Based on the given options, O could be bromine (Br).
O has an atomic number larger than V but smaller than W.
Based on the periodic table, the atomic number of oxygen (O) is 8, which is larger than the atomic number of vanadium (V) (23) and smaller than the atomic number of tungsten (W) (74).
The charge on an L ion is +2.
The charge of +2 indicates that L must lose two electrons to form the ion. Based on the given options, L could be calcium (Ca).
C has five electrons in its outer energy fever.
Carbon (C) has four valence electrons, not five. This contradicts the given statement, so we need to revisit the deductions.
Learn more about carbon on
https://brainly.com/question/19083306
#SPJ1
NEED HELP! An experiment _____. must always be done in a laboratory is best done if only one variable is tested for at a time can be done only by direct observation must always be done in the field
Answers
Answer:
Chemically
in chemistry
The volume of a gas is 654 ml at 6.00oc and 65.3 kpa, what is the volume at 4.00oc and 108.7kpa.
Answers
Therefore, the volume of the gas at 4.00oc and 108.7kpa is 583 ml.
To solve this problem, we need to use the combined gas law, which relates the volume, pressure, and temperature of a gas. The formula for the combined gas law is:
(P1 x V1) / (T1) = (P2 x V2) / (T2)
Where P1, V1, and T1 are the initial pressure, volume, and temperature, respectively, and P2, V2, and T2 are the final pressure, volume, and temperature, respectively.
We can plug in the given values into the formula and solve for V2:
(P1 x V1) / (T1) = (P2 x V2) / (T2)
(65.3 kpa x 654 ml) / (6.00oc + 273.15) = (108.7 kpa x V2) / (4.00oc + 273.15)
Simplifying the equation, we get:
V2 = (65.3 kpa x 654 ml x (4.00oc + 273.15)) / (6.00oc + 273.15) / (108.7 kpa)
V2 = 583 ml
to know more about combined gas law visit:
https://brainly.com/question/30458409
#SPJ11
What is the IUPAC name of the compound shown here?

Answers
Answer:
2-methylpropanoic acid
Explanation:
no probs chief
What is a disadvantage of geothermal energy
Answers
Answer:
it runs the risk of triggering earthquakes. releases harmful gases
what is the molecular formula of first 20 elements
Answers
Answer:
H
He
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Chlorine can bond with fluorine to form ClF. Chlorine can also bond with lithium to form LiCl. Which compound will have a greater partial charge?Question 2 options:A) Both compounds will have the same partial charge.B) ClFC) LiClD) Neither compound will have partial charge.
Answers
In this question, we have two compounds, ClF and LiCl. The first molecule is a covalent compound, since we will not have donation of electrons but instead the sharing of electrons, but in this case, the difference of the electronegativity will cause this molecule to have Polar covalent bond, which will cause partial charges to occur. LiCl is a molecule in which we have a greater difference in electronegativity, which makes this molecule be an ionic molecule, and not covalent. Since ionic compounds have real charges, the only possible answer will be ClF, since they have partial charges. Letter B
What term is used to describe the substance that is totally consumed in a chemical reaction?
Answers
Reactant term is used to describe the substance that is totally consumed in a chemical reaction.
What are reactants?
Reactants are the substances that enter into and are used up in a chemical reaction. They are the starting materials that come together to form the products of the reaction. Reactants are found on the left side of the equation for a chemical reaction, and the products are found on the right side. Reactants are often referred to as substrates, as they are the substances from which the reaction builds the products. All chemical reactions involve the combining of at least two reactants, usually in the presence of a catalyst. Understanding the reactants involved in a reaction is essential to understanding the reaction itself.
Therefore, Reactant term is used to describe the substance that is totally consumed in a chemical reaction.
To learn more about Reactant
Here: https://brainly.com/question/28732327
#SPJ4
What mass in grams of hydrogen is produced by the reaction of 2.0 g of magnesium? (Make sure to balance the reaction first)
Mg(s) + H2O(1) --> Mg(OH)2(s) + H2 (8)
Answers
Answer:
0.164541341 g H2
Explanation:
1) Convert grams to moles by dividing by RMM of Magnesium (24.31g).
2g Mg * (1 mol Mg / 24.31 g Mg) = 0.082270671 mol of Mg
2) Use the balanced equation's ratio of 1 mol Mg: 1 mol H2.
0.082270671 mol of Mg = 0.082270671 mol of H2
3) Convert the mol of H2 back into grams by multiplying by H2's RMM (2 g).
0.082270671 mol of H2 * 2 g H2 = 0.164541341 g H2
* Answer can be rounded to your liking *
When a metal such as copper is heated,it expands. Can anyone tell me what happens to the metal particles as the solid metal expands?
Answers
Answer:
The metal molecules inside start to vibrate faster, causing the solid to change to liquid if exposed to the intense temperature over a long period of time.
Explanation:
As my answer states, but for an explanation, lets say you are boiling an ice cube, it will start to turn into liquid water, that is because it has been exposed to that heat for a long period of time. The same will happen for the metal, if its exposed for long enough, it will turn into a liquid, then can be turned into a gas because the molecules are vibrating so quickly that they start to easily move apart.
Hope this helps, let me know if any more clarification is needed.
How many milliliters of a 0.80mM ammonium hydroxide solution are required to create 2.0L of 0.30mM solution? Do not include units in the answer. Your answer should have two significant figures.
Answers
To make 2.0L of a 0.30mM solution, \(6.4 \times 10^3\) millilitres of a 0.80mM ammonium hydroxide solution are needed.
What is ammonium hydroxide?An aqueous solution containing ammonium (\(NH_4\)) and hydroxide (\(OH^-\)) ions is known as ammonium hydroxide. Because it is a strong base, it totally dissociates in aqueous solutions, resulting in an alkaline reaction.
Ammonium hydroxide is utilised in a wide range of commercial and domestic applications, including the production of cleaning agents. It can be used for cleaning, deodorising, and bleaching, among other things. It serves as a dough conditioner and an
acidity regulator in the food sector.
\(6.4 \times 10^3\)
Let x = 0.80 mM ammonium hydroxide solution in millilitres is needed.
We know that molarity (mM) = moles/L
Given:
\(M_1\) = 0.80 mM
\(M_2\) = 0.30 mM
\(V_2\) = 2.0 L
As a result, we may construct the equation shown below:
(0.80 mM)(x mL) / (1000 mL/L) = (0.30 mM)(2.0 L)
Solving for x, we get:
x = \(6.4 \times 10^3\) mL
In order to produce 2.0L of 0.30mM solution, \(6.4 \times 10^3\) mL of a 0.80mM ammonium hydroxide solution are needed.
To learn more about ammonium hydroxide, visit:
brainly.com/question/31367127
#SPJ1
A __________________ chemical equation shows that the same number of atoms in the reactants are also in the product.
balanced
rearranged
transferred
beautiful
Answers
How many moles are present in 5.67x10^25 atoms of Carbon (C)?
Answers
Answer:
94.155 Moles
Explanation:
Since you are given atoms, to get to moles you need to use the formula I used below. Pretty simple, just plug and chug. Work can be found below.
Hope this helps! :^)
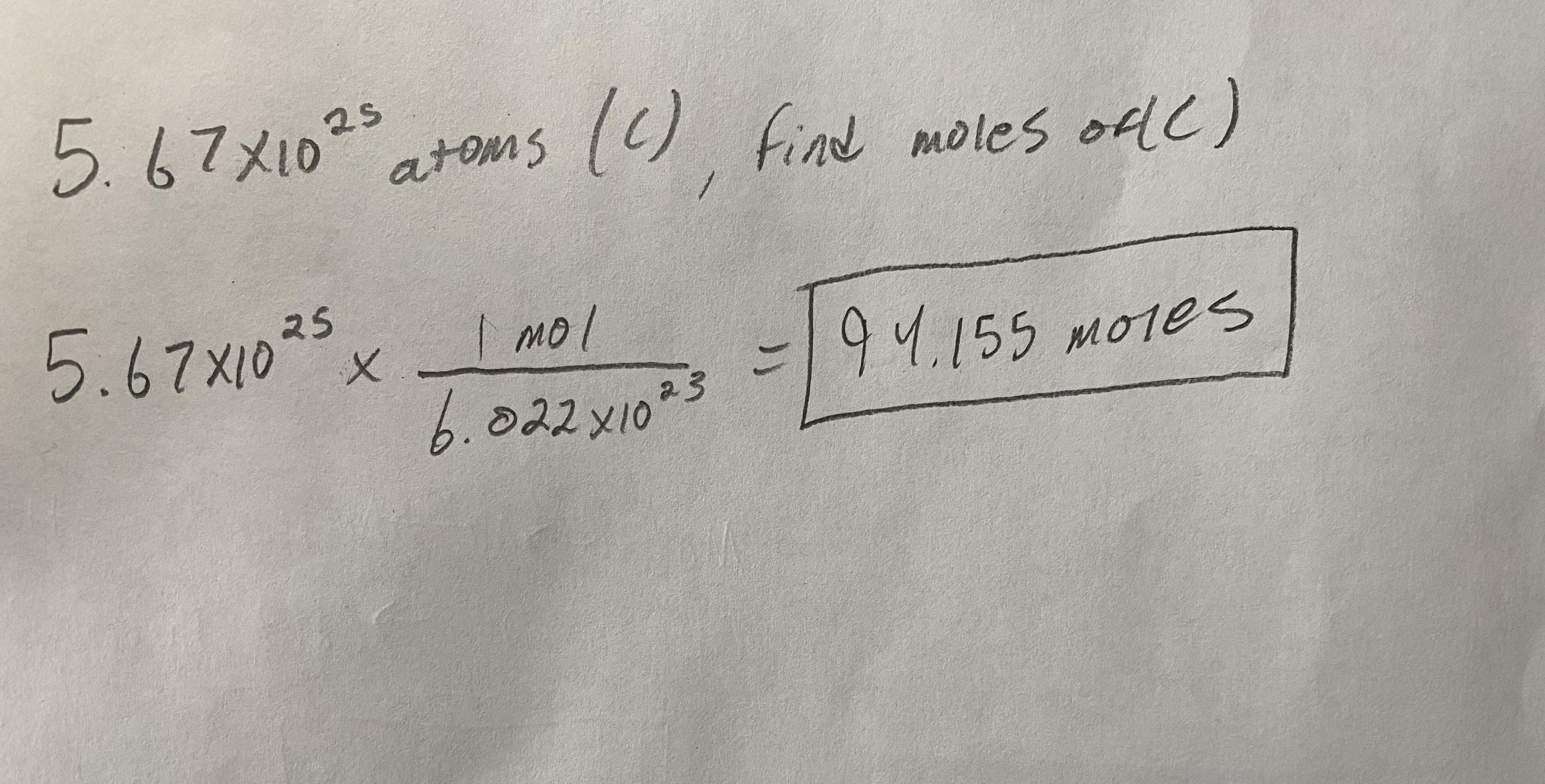
94. 125 moles are present in 5.67x10^25 atoms of Carbon (C).
What is mole?
The mole is a unit of measurement that is comparable to well-known units like pair, dozen, bulk, etc. It offers a precise count of atoms or molecules in a large sample of materials.
The amount of substance that has the same number of discrete entities (atoms, molecules, ions, etc.) as the number of atoms in a sample of pure 12C weighing exactly 12 g is known as a mole.
The word "mole" has a "big mass" or "bulk" sense in Latin, which is congruent with its use as the name for this unit. The mole establishes a connection between bulk mass, which is a simple macroscopic attribute to measure, and the number of atoms, molecules, and other fundamental units, which is a crucial fundamental property.
Therefore, 94. 125 moles are present in 5.67x10^25 atoms of Carbon
(C).
To learn more about mole, refer to the link:
https://brainly.com/question/26416088
#SPJ2
consider the following redox reaction: al(s) hso4-(aq) oh-(aq) → al2o3(s) s2-(aq) h2o(l) what is the coefficient of hso4- when the equation is balanced using the smallest whole numbers?
Answers
The coefficient of HSO₄⁻ in the balanced equation is 1
The coefficient of HSO₄⁻ in the balanced redox reaction can be determined by assigning oxidation numbers and balancing the atoms and charges on both sides of the equation.
In the unbalanced equation: Al(s) + HSO₄⁻(aq) + OH⁻(aq) → Al₂O₃(s) + S²⁻(aq) + H₂O(l)
Let's assign the oxidation numbers:
Al: 0 (unchanged)
H: +1 (unchanged)
S: +6 in HSO₄⁻ and -2 in S²⁻
O: -2 (unchanged)
To balance the equation, we start by balancing the atoms other than hydrogen and oxygen. Since we have two aluminum atoms on the product side, we need to place a 2 in front of Al on the reactant side:
2Al(s) + HSO₄⁻(aq) + OH⁻(aq) → Al₂O₃(s) + S²⁻(aq) + H₂O(l)
Next, we balance the charges by adding electrons (e⁻). On the reactant side, the total charge is -1 from HSO₄⁻ and -1 from OH⁻, making it -2 in total. On the product side, the total charge is -2 from S²⁻. To balance the charges, we need to add two electrons (2e⁻) on the reactant side:
2Al(s) + HSO₄⁻(aq) + 2OH⁻(aq) → Al₂O₃(s) + S²⁻(aq) + H₂O(l) + 2e⁻
Finally, we check the balance of hydrogen and oxygen atoms. On the reactant side, we have 2 hydrogen atoms from HSO₄⁻ and 2 hydrogen atoms from H₂O. On the product side, we have 2 hydrogen atoms from H₂O and 2 hydroxide ions (OH⁻), which account for 2 hydrogen atoms. Hence, the hydrogen atoms are balanced.
Know more about Redox Reaction here:
https://brainly.com/question/28300253
#SPJ11
Stirring will A. Increase a reaction B. Decrease a reaction C. Cause no change D. Create a catalyst
Answers
What does a negative A Hf for a molecule mean?
Answers
Energy was released when the molecule went through a phase change.
Source:
Trust me bro
Answer:
Energy was released when the molecule was formed from its elements
Explanation:
a p e x

The element in group 14, period 3 of the periodic table is classified as a?
1. Metal
2. Noble gas
3. Metalloid
4. nonmetal
Answers
Answer:
It's a Metalloid
Explanation:
Explain in terms of structure and bonding why carbon dioxide has a low boiling point and calcium carbide has a high boiling point
Answers
Answer:
See explanation
Explanation:
Let us consider the two compounds carefully. Carbon dioxide is purely a non-polar molecular compound while the bond between the C2^2- ion and Ca^2+ is ionic thereby making CaC2 an ionic compound.
Recall that ionic compounds have high melting and boiling points due to strong ionic interactions in the structure of the compound.
On the other hand, CO2 is a non-polar substance whose molecules are held together only by weak dispersion forces.
As a result of the reasons outlined above, carbon dioxide has a low boiling point and calcium carbide has a high boiling point.
If something is renewable, then it can be used carelessly. True or false
Answers
If a concentrated solution of potassium bromide is electrolysed, what gas will be produced at the cathode?
Answers
Answer:
Molten lead bromide
Explanation:
PbBr 2(l), is an electrolyte. During electrolysis: Pb 2+ ions gain electrons at the cathode and become Pb atoms. Br - ions lose electrons at the anode and become Br atoms, which pair up to form Br 2 molecules.
BrF5 oxidation number
Answers
Answer:
+5
Explanation:
hope this helps! :)
Aspirin is made commercially by adding acetic anhydride to an aqueous solution of salicylic acid. if 200. g of acetic anhydride is added to 138 g of salicylic acid what is the theoretical yield?
Answers
So, the theoretical yield of aspirin in this reaction is 180.16 grams.To determine the theoretical yield of aspirin, we need to calculate the limiting reactant and use stoichiometry.First, let's find the number of moles for acetic anhydride and salicylic acid:
Molar mass of acetic anhydride (C4H6O3) = 102.09 g/molNumber of moles of acetic anhydride = mass / molar mass = 200 g / 102.09 g/mol = 1.96 molMolar mass of salicylic acid (C7H6O3) = 138.12 g/mol
Number of moles of salicylic acid = mass / molar mass = 138 g / 138.12 g/mol = 1 mol
Next, we need to compare the moles of each reactant to determine the limiting reactant. The reactant with fewer moles will limit the amount of product that can be formed. In this case, salicylic acid is the limiting reactant because it has fewer moles (1 mol) compared to acetic anhydride (1.96 mol).
To know more about aspirin, visit :-
https://brainly.com/question/14988384
#SPJ11
State how each trend changes (increase/decrease) within a group
Answers
Answer:
I don't know which group you mean, but this is
trends in atomic radius in periods and in groups.
Trend within periods: decreases from left to right
Trend within groups: increases from top to bottom
Trends in ionization energy in periods and in groups.
Trend within periods: increases from left to right
Trend within groups: decreases from top to bottom
Trends in electronegativity in periods and in groups.
Trend within periods: increases from left to right
Trend within groups: decreases from top to bottom
I hope this helped answer your question. :) <3
Explanation:
a solution of acetic acid that has a concentration of 0.10 moles per liter has a ph of 2.87. what is the likely ph of a 0.10 mole per liter solution of the conjugate base sodium acetate?
Answers
0.10 moles per liter solution of the conjugate base sodium acetate is likely to have a pH greater than 7.
Is the pH of a 0.10 mole per liter solution of the conjugate base sodium acetate likely to be acidic or basic?When acetic acid (CH3COOH) donates a proton, it forms its conjugate base, acetate ion (CH3COO-). In the given scenario, the acetic acid solution has a pH of 2.87, indicating acidity. The lower pH value suggests a higher concentration of H+ ions. As a weak acid, acetic acid partially dissociates, releasing H+ ions and acetate ions. When sodium acetate (CH3COONa) dissolves in water, it completely dissociates into sodium ions (Na+) and acetate ions. The presence of acetate ions (the conjugate base) from sodium acetate will react with the excess H+ ions in the solution, shifting the equilibrium towards the formation of acetic acid and water. This process, called the hydrolysis of salts, will consume the H+ ions, thereby increasing the pH of the solution. Consequently, the 0.10 mole per liter solution of sodium acetate is likely to have a pH greater than 7, making it basic.
Learn more about pH
brainly.com/question/2288405
#SPJ11
Name the following covalent compound: CCl3
monocarbon trichloride
carbon chloride
carbon trichloride
tricarbon monochloride
Answers
Answer:
The name for CCl3 is carbon tetrachloride.