Why are hydrogen bonds the strongest of the intermolecular forces.
Answers
Hydrogen bonds are also directional, meaning that they have a specific orientation in space. This directional nature allows for a strong interaction between two atoms or molecules, which increases the strength of the bond. Overall, the combination of high polarity, small size, and directional nature makes hydrogen bonds the strongest of the intermolecular forces.
Intermolecular forces are forces that exist between atoms and molecules and hold them together. These forces affect the physical properties of substances such as boiling point, melting point, and viscosity. Hydrogen bonds are one of the strongest intermolecular forces, and this can be attributed to several factors.The strength of a hydrogen bond can be explained by its electrostatic nature. A hydrogen bond is formed between a hydrogen atom bonded to an electronegative atom and another electronegative atom. This creates a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom. The partial charges attract each other and create a bond between the two atoms. This electrostatic attraction is strong, which is why hydrogen bonds are strong.One factor that contributes to the strength of hydrogen bonds is the high polarity of the bond. Since hydrogen has a low electronegativity, it has a high electron density, making it a good candidate to form hydrogen bonds. Another factor that contributes to the strength of hydrogen bonds is the small size of hydrogen atoms. The small size of the hydrogen atom allows it to be closer to the other atom, which increases the electrostatic attraction and makes the bond stronger.Hydrogen bonds are also directional, meaning that they have a specific orientation in space. This directional nature allows for a strong interaction between two atoms or molecules, which increases the strength of the bond.Overall, the combination of high polarity, small size, and directional nature makes hydrogen bonds the strongest of the intermolecular forces.
To Know more about Hydrogen bonds visit:
brainly.com/question/31139478
#SPJ11
Related Questions
Table salt is sodium chloride and has the chemical formula of NaCl. What is the ratio of sodium atoms to chlorine atoms? (8.5D)
Answer
А 2:1
B
1:1
С
1:2
D
3:1
Answers
Answer:
Explanation:Table salt is sodium chloride and has the chemical formula of NaCl. What is the ratio of sodium atoms to chlorine atoms? (8.5D)
Answer
А 2:1
B
1:1
С
1:2
D
3:1
Pam's family is clearing some land, and there is a large boulder sitting on top of the ground. Even with the entire family pushing on it, they cannot mov
it. Which type of friction exists between the boulder and the ground

Answers
Answer:
d
Explanation:
I believe the answer to this question is d , I believe this as the question doesn't explain properly the surface under the boulder. it is questionable as the question is not clear though I believe this as there must be friction under the boulder stopping it from moving.
A container initially holds 5.67 x 10^-2 mol of propane and has a volume of V1. The volume of the container was increased by adding an additional 2.95 x 10^-2 mol if propane to the container, so that the container has a final volume of 1.93 L. If the temperature and pressure are constant, what was the initial volume of the container?
Answers
Answer:
Initial volume of the container (V1) = 1.27 L (Approx)
Explanation:
Given:
Number of mol (n1) = 5.67 x 10⁻²
Number of mol (n2) = (5.67 +2.95) x 10⁻² = 8.62 x 10⁻²
New volume (V2) = 1.93 L
Find:
Initial volume of the container (V1)
Computation:
Using Avogadro's law
V1 / n1 = V2 / n2
V1 / 5.67 x 10⁻² = 1.93 / 8.62 x 10⁻²
V1 = 10.9431 / 8.62
Initial volume of the container (V1) = 1.2695
Initial volume of the container (V1) = 1.27 L (Approx)
Answer: 1.27 L
Explanation:
First, calculate the final number of moles of propane (n2) in the container.
n2 = n1 + nadded = 5.67 × 10^−2 mol + 2.95 × 10^−2 mol = 8.62 × 10^−2 mol
Rearrange Avogadro's law to solve for V1.
V1 = V2 × n1 / n2
Substitute the known values of n1, n2, and V2,
V1 = 1.93 L × 5.67 × 10^−2 mol / 8.62 × 10^−2 mol = 1.27 L
what element has 10 protons and 12 electrons and 10 neutrons
Answers
Answer:
Magnesium Ion
Explanation:
This is a Magnesium ion. There are 12 protons from the magnesium atom. There are 10 electrons because 12-2=10. There are 12 neutrons because 24-12=12
10) element x is in group I of the periodic table. X reacts with element Y to form an ionic compound. Which equation shows the process that takes place when X forms ions

Answers
We require the equation to understand the process that occurs when X reacts with Y to form an ionic compound.The chemical equation for the formation of the ionic compound between X and Y would be: X + Y → XYwhere X represents the alkali metal in group I and Y represents a non-metal that is most likely in group VII. This equation represents the process of how the two elements react with each other to create an ionic compound.
Element X is found in group I of the periodic table, which means it belongs to the alkali metal group. Alkali metals are well-known for their reactivity, with the exception of lithium, which is the least reactive alkali metal. Alkali metals react with other elements to form ionic compounds. Let’s take a closer look at this process.Element X reacts with Element Y to create an ionic compound, which means that Element X becomes an ion in the process. Since Element X is an alkali metal, it has only one valence electron.
To form a positive ion, it loses this valence electron.Element Y, on the other hand, is probably a non-metal since it’s reacting with an alkali metal. Non-metals, unlike alkali metals, have a high electronegativity. As a result, they have a tendency to take electrons from other elements in order to complete their valence shells.
As a result, Element Y gains an electron in this instance.Since X loses its valence electron and Y gains an electron, X becomes a positive ion and Y becomes a negative ion. The resulting ionic compound is formed by the attractive forces between the positive and negative ions. The formula of the ionic compound is determined by the ratio of the ions present.
for more questions on ionic
https://brainly.com/question/30373783
#SPJ8
How many moles of sodium bicarbonate will react with the number of moles citric acids you determined in #1
1*10^-3/1=1mol/3
Answers
The number of moles of sodium bicarbonate that will react with the number of moles of citric acid determined in #1 is equal to the number of moles of citric acid divided by three.
This is because the reaction between sodium bicarbonate and citric acid is a 3:1 molar ratio, meaning that for every three moles of sodium bicarbonate, one mole of citric acid is consumed . Therefore, using the given value of 1*10^-3/1=1mol/3 for the number of moles of citric acid, we can calculate the number of moles of sodium bicarbonate as follows:
n (NaHCO3) = n (C6H8O7) / 3n (NaHCO3) = 1*10^-3/1 / 3n (NaHCO3) = 0.333*10^-3 molHence, 0.333*10^-3 mol of sodium bicarbonate will react with 1*10^-3/1 mol of citric acid.
About Citric acidCitric acid is a weak organic acid with the chemical formula HOC(CH₂CO₂H)₂ which is found in the leaves and fruits of plants of the genus Citrus. This compound is a good and natural preservative, besides being used as a sour taste enhancer in food and soft drinks.
Learn More About Citric acid at https://brainly.com/question/30760037
#SPJ11
When 5.101 g of solid magnesium sulfate heptahydrate, MgSO4·7 H2O, was dissolved in 48.709 g of water in a coffee cup calorimeter, the temperature of the solution decreased from 20.7 °C to 19.3 °C. Calculate qreaction (in J) for this process.
Answers
the value of qreaction (in J) for the given process is -321.54 J.
For the given problem, the mass of the solution, ms, can be determined as:
ms = 5.101 g + 48.709 g= 53.810 g
The specific heat of the solution, s, is considered to be equal to 4.18 J/g °C (specific heat of water).The change in temperature of the solution, ΔT, can be determined as:
ΔT = final temperature - initial temperature= 19.3 °C - 20.7 °C= -1.4 °C (negative sign indicates that the temperature of the solution has decreased during the process)
Thus, the value of qreaction can be determined as:
qreaction = msΔT
= 53.810 g × 4.18 J/g °C × (-1.4 °C)
= -321.54 J (negative sign indicates that the heat has been absorbed by the solution)
Therefore, the value of qreaction (in J) for the given process is -321.54 J.
learn more about calorimeter here
https://brainly.com/question/24167789
#SPJ11
How does the enthalpy of photosynthesis compare to the enthalpy of cellular respiration?
A. Respiration releases twice as much energy from glucose as photosynthesis takes to form glucose.
B. Photosynthesis has a much higher enthalpy than cellular respiration.
C. Respiration stores three times as much energy as photosynthesis.
D. Respiration has an equal enthalpy to cellular respiration
Answers
Respiration has an equal enthalpy to cellular respiration. The correct option is D.
What is enthalpy?A thermodynamic system's enthalpy is the sum of its internal energy and the product of its volume and pressure.
It is a state function utilized in numerous measurement techniques in chemical, biological, and physical systems at constant pressure, which the large ambient atmosphere conveniently provides.
At its initial stage, cellular respiration combines the information of photosynthesis, and photosynthesis uses the results of cellular respiration.
The processes of cellular respiration and photosynthesis have the same enthalpy.
Thus, the correct option is D.
For more details regarding enthalpy, visit:
https://brainly.com/question/16994235
#SPJ1
PLEASE HELP!!!
How many particles Fe2O3 will produce in an aqueous solution (in water).
Question 11 options:
2
3
4
5
Answers
Hydrolysis occurs when Fe2O3 is dissolved in water, resulting in the formation of Fe(OH)3 and H+ ions. For every Fe in Fe2O3, there are 1.5 O. Thus, the iron content of Fe2O3 is reduced.
An aqueous solution is what?Water in the liquid state serves as the solvent in an aqueous solution. In other words, water molecules surround and integrate solute (dissolved) ions and molecules into their web of bonds. Following that, the dissolved species dispersed all over the water.
Why is water referred to as an aqueous solution?Aqueous solutions are made up of water and one or more dissolved materials. Solids, gases, or other liquids can all dissolve in an aqueous solution.
To know more about Hydrolysis visit:-
brainly.com/question/10840252
#SPJ1
Question:
How Many Particles Fe2O3 Will Produce In An Aqueous Solution (In Water).
what is removed during the formation of nucleic acid polymers?
Answers
Answer:
inside cells are removed
what is the pH of a solution with [H+] = 1.25 x 10^-10M?
Answers
pH = -log[H+]
Given [H+] = 1.25 x 10^-10 M:
pH = -log(1.25 x 10^-10)
pH = -log(1.25) + log(10^-10)
pH ≈ -9 + (-10)
pH ≈ -19
Therefore, the pH of the solution with [H+] = 1.25 x 10^-10 M is approximately -19.
Answer:
9.90
Explanation:
Given [H+] = 1.25 x 10^-10 M, we can calculate the pH using the formula:
pH = -log10([H+])
pH = -log10(1.25 x 10^-10)
Using logarithmic properties:
pH = -log10(1.25) - log10(10^-10)
Since log10(10^-10) is equal to -10:
pH = -log10(1.25) - (-10)
pH = -log10(1.25) + 10
Now, evaluating the logarithm using a calculator:
pH = -0.0969 + 10
pH = 9.9031
Therefore, the pH of the solution with [H+] = 1.25 x 10^-10 M is approximately 9.9031. Rounding it to two decimal places, the pH is approximately 9.90.
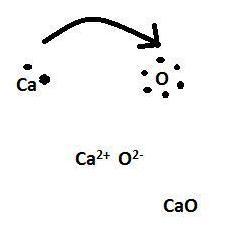
the correct lewis dot structure for CaO?
Answers
Answer:
Here you go.
Explanation:
Hopefully it is not confusing.
What is the functional formula of ketones?
Answers
R–C(=O)–R is the functional formula of ketones .
What are compounds ?
A compound is a substance consisting of two or more elements chemically combined in a fixed ratio. They differ from mixtures consisting of two or more substances physically mixed without chemical bonding. Compounds have their own physical and chemical properties that differ from those of individual elements. For example, the compound water (H2O) has different properties (boiling point, density, solubility, etc.) than its components hydrogen (H2) and oxygen (O2).
Compounds are formed by chemical reactions in which atoms of different elements combine to form new substances. The formation of a compound can be represented by a chemical formula that indicates the type and number of atoms in the compound.
To know more about compounds , click the link below ;
brainly.com/question/26487468
#SPJ4
Why do you think carbon monoxide poisoning caused ms. beauregard's body to turn bright cherry red?
Answers
Carbon monoxide poisoning caused ms. beauregard's body to turn bright cherry red because of a specific interaction between carbon monoxide and haemoglobin in the blood.
Carbon monoxide (CO) poisoning can cause a person's body to turn bright cherry red due to a condition called "cherry-red coloration." This phenomenon occurs as a result of a specific interaction between carbon monoxide and hemoglobin in the blood.
Hemoglobin is the protein responsible for carrying oxygen throughout the body. It binds with oxygen in the lungs and releases it to the body's tissues. However, carbon monoxide has a strong affinity for haemoglobin and forms a stable complex called carboxyhaemoglobin (COHb) when it enters the bloodstream.
The formation of carboxyhaemoglobin is problematic because it reduces the oxygen carrying capacity of the blood. Carbon monoxide binds to haemoglobin with much greater affinity than oxygen, leading to the displacement of oxygen from haemoglobin. This means that less oxygen is available to be transported to the body's tissues.
When a significant amount of carboxyhaemoglobin is present in the blood, it can result in oxygen deprivation, tissue hypoxia, and potentially fatal consequences. The bright cherry-red coloration observed in carbon monoxide poisoning occurs because carboxyhemoglobin has a bright red color. This coloration affects the entire body, including the skin, mucous membranes, and tissues..
If carbon monoxide poisoning is suspected, it is essential to seek immediate medical attention as it can be life-threatening. Prompt treatment involves removing the person from the carbon monoxide source and providing them with supplemental oxygen to restore oxygen levels in the blood and tissues.
To know more about carbon monoxide here
https://brainly.com/question/30225838
#SPJ4
When it gets cold outside, a car's tire pressure can decrease. What might be happening to the gas molecules in the tire to cause the decrease in pressure?
Answers
This can be explained by Boyle’s Law (P1 P2 = V1 V2), where pressure is inversely proportional to volume (at a constant temperature).
What mass of water is required to dissolve 175 g KNO3 (potassium Nitrate) to produce a 32.25 m solution?
Answers
ANSWER
The mass of water is 0.0536 kg
STEP-BY-STEP EXPLANATION;
Given information
The mass of KNO3 = 175g
The molarity of the solution = 32.25 M
The molality formula is given below as
\(\text{ Molality = mole of solute }\div\text{ kg of solvent}\)The first step is to find the mole of the solute using the below formula
\(\text{ Mole = mass }\div\text{ molar mass}\)Recall, the molar mass of KNO3 is 101.1032 g/mol
\(\begin{gathered} \text{ Mole = 175 }\div\text{ 101.1032} \\ \text{ Mole = 1.731 moles} \end{gathered}\)The second step is to find the mass of water using the molality formula
\(\begin{gathered} \text{ Molality = mole of solute }\div\text{ kg of solvent} \\ 32.25\text{ = 1.731}\div\text{ kg of solvent} \\ \text{ cross multiply} \\ \text{ 1.731 = 32.25 }\times\text{ Kg of solvent} \\ \text{ kg of solvent = 1.731 }\div\text{ 32.25} \\ \text{ kg of solvent = 0.0536 kg} \end{gathered}\)Hence, the mass of water is 0.0536 kg
Answer the question in attachment and can I ask from which country you are.
I am new on this app.
✌️✌️☺️☺️

Answers
Answer:
1: exothermic
2:hydrated iron (III) oxide
3:reactants
4:precipitate
5: hydrogen gas
Answer:1.exothermic
2.hydrated iron (III) oxide
3.reactants
4.precipitate
5. hydrogen gas
A piece of barium has a volume of
4.00 cm3. The density of barium
is 3.62 g/cm3. What is the mass
of the sample of barium?
Answers
These three variables have a common equation:
\(D=\dfrac{m}{V}\)
Solving the Question:We're given:
V = 4.00 cm³D = 3.62 g/cm³Isolate m in the equation:
\(m=DV\)
Plug these values into the equation:
\(m=(3.62\frac{g}{cm^3})(4.00 cm^{3})\\\\m=14.48g\)
AnswerThe mass of the sample of barium is 14.48 g.
Two gases are placed in a sealed flask and allowed to react. Which statement is true about the concentrations of the reactants and products when this closed system reaches dynamic equilibrium?
Answers
They will not necessarily be equal, but they will be constant.
What is dynamic equilibrium?In reversible reactions, dynamic equilibrium refers to a state in which the rate of the forward reaction is equal to that of the backward reaction.
Consdier the reaction below: A + B --> C + D
If the reaction is in dynamic equilibrium, the rate of formation of A and B will be the same as the rate of formation of C and D. However, this does not mean that the concentrations of the reactants and the products will be the same.
Since the rate of forward and backward reactions are equal, it means that the concentration of each of the species will be constant.
Thus, the above reaction is applicable to that of two reacting gases placed in a sealed flask and allowed to reach a dynamic equilibrium.
More on dynamic equilibrium can be found here: https://brainly.com/question/2363073
#SPJ1
They will be equal and constant. They will be equal but continuously changing. They will not necessarily be equal, and they will be continuously changing. They will not necessarily be equal, but they will be constant.
A 150 ml sample of a gas is heated from 35 °C to 85°C at constant pressure. What is the final volume? Please list known and unknown quantities, units, formula used in the problem and show substituted values in the problem
Answers
Answer:
thats hard ummm
Explanation:
yeah sorry i can't answer that
but heres an example: just go to this site it should help
Charles' Law Calculator
hope this works
Calculate the mass of ammonium sulfide (NH4)2S in 3.00 L of a 0.0200 M solution.
Answers
The mass of (NH4) 2S in the solution is : Mass = 0.0600 mol × 60.08 g/mol = 3.60 g.
The given molarity and volume of the solution can be used to calculate the number of moles of ammonium sulfide (NH4)2S.Then, the number of moles can be converted to mass using the molar mass of (NH4)2S.Mass of ammonium sulfide (NH4)2S in 3.00 L of a 0.0200 M solution is given by : Mass = moles × molar mass.The number of moles of (NH4)2S can be found using the equation:Molarity = Number of moles / Volume.Rearranging this equation, we get:Number of moles = Molarity × Volume Number of moles of (NH4)2S = 0.0200 M × 3.00 L.Number of moles of (NH4)2S = 0.0600 mol.The molar mass of (NH4)2S can be calculated by summing the molar masses of ammonium (NH4) and sulfide (S) ions.Molar mass of (NH4)2S = (2 × Molar mass of NH4) + Molar mass of S= (2 × 14.01 g/mol) + 32.06 g/mol= 60.08 g/mol.
For more question on mass
https://brainly.com/question/1838164
#SPJ8
How can a soda can and illumine foil be the same mass?
Answers
Answer:
Foil is made from the same material as soda cans (aluminum), but since it's most often contaminated with food waste or combined with plastic (like with yogurt tops), there's no guarantee you can recycle it with your aluminum cans.
Explanation:
Mark me brainly please
calculate ph for this strong base solution: 8.2×10−2 m koh .
Answers
The pH for a strong base solution is calculated using the formula; pH = 14 - pOH. We know that KOH is a strong base, therefore, we can use this formula to calculate the pH
Given concentration of KOH To find the pH of a strong base solution, we first need to find the concentration OH- ions present in the solution. As KOH is a strong base, it completely dissociates in water to form KOH molecules and hydroxide ions, as shown below ;KOH → K+ + OH-From the given information, the concentration of KOH in the solution is 8.2 × 10−2 M. As the KOH is completely dissociated in water, the concentration of hydroxide ions will also be equal to 8.2 × 10−2 M.To find the pOH of the solution, we can use the formula; pOH = - log [OH-]Where, [OH-] is the concentration of hydroxide ions in the solution .pOH = - log [8.2 × 10−2]pOH = 1.09Now, using the formula pH = 14 - pOH, we can find the pH of the solution. pH = 14 - 1.09pH = 12.91Therefore, the pH of the 8.2 × 10−2 M KOH solution is 12.91.
The pH is a measure of the acidity or basicity of a solution. It is a measure of the concentration of hydrogen ions (H+) present in the solution. pH is defined as the negative logarithm (base 10) of the hydrogen ion concentration in moles per liter (molarity).pH = -log[H+]The pOH is defined as the negative logarithm (base 10) of the hydroxide ion concentration in moles per liter (molarity).pOH = -log[OH-]The pH and pOH are related by the equation:pH + pOH = 14A neutral solution has a pH of 7. An acidic solution has a pH less than 7. A basic solution has a pH greater than 7.KOH is a strong base. A strong base is one that is completely ionized (dissociated) in an aqueous solution. The dissociation of KOH can be represented by the following equation KOH → K+ + OH-The concentration of hydroxide ions (OH-) in a 8.2 × 10−2 M KOH solution is equal to the concentration of KOH (8.2 × 10−2 M).pOH = -log[OH-] = -log(8.2 × 10−2) = 1.09pH = 14 - pOH = 14 - 1.09 = 12.91Therefore, the pH of a 8.2 × 10−2 M KOH solution is 12.91.
To know more about pH Visit;
https://brainly.com/question/15289741
#SPJ11
What do you understand by the terms radial node and nodal plane, as applied to AO wavefunctions? Illustrate your answer using the 2s and 2p AOs. Explain why radial nodes arise from the radial part of the wavefunction, whereas nodal planes arise from the angular part of the wavefunction
Answers
In the context of atomic orbital (AO) wavefunctions, the terms "radial node" and "nodal plane" refer to different aspects of the wavefunction's behavior.
A radial node is a region in the AO wavefunction where the probability of finding an electron is zero along the radial direction. In other words, it represents a spherical shell where the electron is unlikely to be found. The number of radial nodes is determined by the principal quantum number (n) of the orbital. For example, the 2s orbital has one radial node, while the 2p orbital has no radial nodes.
On the other hand, a nodal plane is a flat plane within the AO wavefunction where the probability of finding an electron is zero along a particular direction. It represents a surface that divides the orbital into two regions of opposite phases. The number of nodal planes is determined by the angular quantum numbers (l and m) of the orbital. For example, the 2s orbital has no nodal planes, while the 2p orbital has one nodal plane (the xz or yz plane).
Radial nodes arise from the radial part of the wavefunction because they depend on the distance from the nucleus. The radial part determines the distribution of the electron density as a function of distance, and the nodes correspond to regions where the density drops to zero.
On the other hand, nodal planes arise from the angular part of the wavefunction because they depend on the orientation and shape of the orbital. The angular part describes the angular distribution of the electron density around the nucleus, and the nodal planes correspond to regions where the phase of the wavefunction changes sign.
In summary, radial nodes are related to the distance from the nucleus and arise from the radial part of the wavefunction, while nodal planes are related to the orientation and shape of the orbital and arise from the angular part of the wavefunction. The 2s orbital has one radial node and no nodal planes, while the 2p orbital has no radial nodes and one nodal plane.
learn more about radial node here
https://brainly.com/question/31829965
#SPJ11
I need help with this Asap. Best answer gets brainiest, also how is your day.

Answers
A precipitate is formed when the two solutions, barium nitrate, Ba(NO3)2, and phosphoric acid, H3PO4, are
mixed. Identify the correct products for this reaction.
A)
BaHa & NPO4
B)
BazN2 & HNO3
Baz(PO4)2 & NO2
D)
Baz(PO4)2 & HNO3
Answers
d. Ba₃(PO₄)₂(s)+6HNO₃(aq)
Further explanationDouble-Replacement reactions. Happens if there is an ion exchange between two ion compounds in the reactant to form two new ion compounds in the product
To predict whether this reaction can occur or not is one of them, the precipitation reaction. A precipitation reaction occurs if two ionic compounds which are dissolved reacted to produce one of the products of the ion compound does not dissolve. Formation of these precipitating compounds that cause reactions can occur
Because A precipitate is formed, then compounds of CO₃²⁻ and PO₄³⁻ except for Compounds of Li +, Na +, K +, Rb +, Cs +, and NH₄ + are generally included in the insoluble compound
So the reaction :
3Ba(NO₃)₂(aq)+2H₃PO₄(aq)⇒Ba₃(PO₄)₂(s)+6HNO₃(aq)
A precipitate is formed ⇒ Ba₃(PO₄)₂
A cat has a mass of 8 kg while in
Frankfort, Kentucky (elevation: 509
feet). In Staten Island, New York
(elevation: 161 feet), how much
mass would the cat have?
A. more than in Frankfort
B. less than in Frankfort
C. the same as in Frankfort
Answers
Answer:
C
Explanation:
Remark
The mass would be the same everywhere, not only in Frankford Kentucky.
It's the weight or gravitational pull that changes.
please answer this 1. rana wants to buy shirts for summer.should she buy cotton shirts or shirts made of synthetic material ? advise rana, give ur reason...
Answers
Answer:
Cotton Shirts
Explanation:
I would think that the cotton shirts would be more appropriate form of of clothing for summer.
Cotton has tiny pores that enable the sweat to evaporate off your skin, and hence keep you cooler. At the same time it absorbs this sweat, allowing easy evaporation. Synthetic fibers hold heat in, and do not allow sweat to evaporate.
It would be that Rana should buy cottons shirts for summer.
If some paper and/or paper-like materials ignite and cause a very small fire at your lab table, you should?
Answers
Explanation:
For an effective use of a laboratory with protection and safety for all, there must be rules and policies that must be strictly disseminated and followed by anyone who uses a laboratory for a specific purpose, such as the use of specific clothes and goggles.
However, in the event of a small fire, the ideal is to notify the laboratory instructor, and exit the emergency exits in an orderly and quick manner.
If some materials ignite and cause a small fire you should c. pour water on it or use wet paper towels to put it out.
In the lab there are many guidelines and precautions that must be taken to ensure that things like fire do not happen but sometimes it cannot be helped.
If some materials like paper or paper-like materials ignite and cause a small fire you should put it out quickly by:
pouring water on it or,using wet towels because the fire would be very small.In conclusion, put the fire out as soon as possible using water or a small paper towel so that it doesn't spread.
Find out more at https://brainly.com/question/3409761.
Options for this question include:
a. sound the building alarm and evacuate the area
b. sound the building alarm and then use the fire extinguisher
c. pour water on it or use wet paper towels to put it out
d. run out of the room
A. O CN
B. O NH4+
C. O s2-
D. O Cro 2-
Which of the following is an oxyanion?