Why does ether or petrol have a cooling effect when it touches the hand. Just curious
Answers
During evaporation, particles of the liquid absorb energy from the surface of the palm to compensate for the loss of energy, making the surroundings cool. The acetone, petrol or perfume take this latent heat of vaporisation from our palm. The palm loses heat and feels cold.
Answer:
When we put some acetone or petrol or perfume on our palm, it evaporates. During evaporation, particles of the liquid absorb energy from the surface of the palm to compensate for the loss of energy, making the surroundings cool. This results in the palm becoming colder.
Explanation:
Related Questions
what is the percent composition of salicylic acid?
Answers
The percent composition of salicylic acid is C7H6O3, or 60.87%C, 4.4%H, and 34.75%O
What is the mass percentage of cl in hydrochlorothiazide, c₇h₈cln₃o₄s₂? provide an answer to two decimal places.
Answers
Hydrochlorothiazide, C₇H₈ClN₃O₄S₂, mass percentage of Cl in hydrochlorothiazide is 11.93% ≈ 12.
What is mass percentage?The concentration of an element in a compound or element of a combination can be expressed as a mass percentage. The mass percentage is calculated by multiplying the result by 100% after dividing the combined mass by the masses of each component.
Molecular mass of Cl = 35.5
Molecular mass of C₇H₈ClN₃O₄S₂
= 7×2 + 8×1 + 35.5×1 + 14×3 + 16×4 + 32×2
Molecular mass of C₇H₈ClN₃O₄S₂ = 84 + 8 + 35.5 + 42 + 64 + 64
Molecular mass of C₇H₈ClN₃O₄S₂ = 297.5
You can express the mass percentage of chlorine in hydrochlorothiazide as follows:
Mass percentage = 35.5/297.5×100
Mass percentage = 11.93% ≈ 12
Hence, the mass percentage of of Cl in hydrochlorothiazide, C₇H₈ClN₃O₄S₂ is 11.93% ≈ 12.
Read more about mass percentage visit:
https://brainly.com/question/16885872
#SPJ4
How many grams of aluminum is produced when 82.4 grams of aluminum chloride
decomposes into chlorine gas and aluminum metal?
2AICI: - 2 Al + 3C12
Answers
Answer:
16.6 g of Al are produced in the reaction of 82.4 g of AlCl₃
Explanation:
Let's see the decomposition reaction:
2AlCl₃ → 2Al + 3Cl₂
2 moles of aluminum chloride decompose to 2 moles of solid Al and 3 moles of chlorine gas.
We determine the moles of salt:
82.4 g . 1mol/ 133.34g = 0.618 moles
Ratio is 2:2. 2 moles of salt, can produce 2 moles of Al
Then, 0.618 moles of salt must produce 0.618 moles of Al.
Let's convert the moles to mass → 0.618 mol . 26.98g /mol = 16.6 g
If 2-chloro-2-methylbutane has a density of 0. 866 g/ml. How do you expect it to sit in the separation?
Answers
We expect 2-chloro-2-methylbutane to sit at the bottom of the separation setup due to its higher density compared to water.
The density of 2-chloro-2-methylbutane is given as 0.866 g/ml. The density of a substance helps us determine how it will sit or behave in a separation. In this case, since the density of 2-chloro-2-methylbutane is greater than 1 g/ml (the density of water), it will be more dense than water.
When two immiscible liquids with different densities are placed together, the denser liquid will sink below the less dense liquid. In this case, 2-chloro-2-methylbutane will sink below water if they are placed in a separation setup.
To know more about
https://brainly.com/question/20922015
#SPJ11
There are ___________ forces of attraction between particles in the gas state. Use the following word bank to help you.
Answers
Answer:
Different
Explanation:
how to calculate heat of neutralization of hcl and naoh
Answers
The heat of neutralization for the reaction between HCl and NaOH is -697 kJ/mol
To calculate the heat of neutralization between hydrochloric acid (HCl) and sodium hydroxide (NaOH), you can follow these steps:
Determine the balanced equation for the neutralization reaction between HCl and NaOH.The balanced equation is as follows : HCl + NaOH → NaCl + H₂O
This equation represents the reaction between one mole of HCl and one mole of NaOH, forming one mole of NaCl (sodium chloride) and one mole of water (H₂O).
Find the molar enthalpy of formation (∆Hf) for NaCl and H₂O. This value represents the enthalpy change when one mole of the compound is formed from its constituent elements in their standard states.Look up the ∆Hf values in a reliable reference source or database.
The ∆Hf for NaCl is -411 kJ/mol.
The ∆Hf for H₂O is -286 kJ/mol.
Determine the stoichiometric coefficients from the balanced equation. In this case, the stoichiometric coefficient for NaCl and H₂O is both 1.Calculate the heat of neutralization (∆H) using the formula:∆H = ∆Hf(NaCl) + ∆Hf(H₂O)
Since the stoichiometric coefficients for NaCl and H₂O are both 1, you simply add their respective ∆Hf values.
∆H = -411 kJ/mol + (-286 kJ/mol)
∆H = -697 kJ/mol
The heat of neutralization for the reaction between HCl and NaOH is -697 kJ/mol. The negative sign indicates that the reaction is exothermic, meaning it releases heat.
Thus, ∆H = -697 kJ/mol.
To learn more about neutralization reaction :
https://brainly.com/question/23008798
#SPJ11
What is the difference between fumarate and fumaric acid?
Answers
Answer:
the difference is fumaric acid the carboxylic acid groups are trans (E) and in maleic acid they are cis (Z)
Explanation:
and Fumarate is an intermediate in the citric acid cycle used by cells to produce energy in the form of adenosine triphosphate (ATP) from food
how many moles of H2SO4 need to react with 10.0 mol of iron (III) hydroxide
Answers
Answer:
30.0 moles of H2SO4 are needed to react with 10.0 moles of Fe(OH)3.
Explanation:
The balanced chemical equation for the reaction between H2SO4 and Fe(OH)3 is: Fe(OH)3 + 3H2SO4 → Fe2(SO4)3 + 3H2O According to the equation, 1 mole of Fe(OH)3 reacts with 3 moles of H2SO4. Therefore, to determine how many moles of H2SO4 are needed to react with 10.0 mol of Fe(OH)3, we need to use the mole ratio between Fe(OH)3 and H2SO4: 10.0 mol Fe(OH)3 x (3 mol H2SO4 / 1 mol Fe(OH)3) = 30.0 mol H2SO4.
Therefore, 30.0 moles of H2SO4 are needed to react with 10.0 moles of Fe(OH)3.
what does this graph represent?
A. speed
B. velocity
C. acceleration
D. momentum?
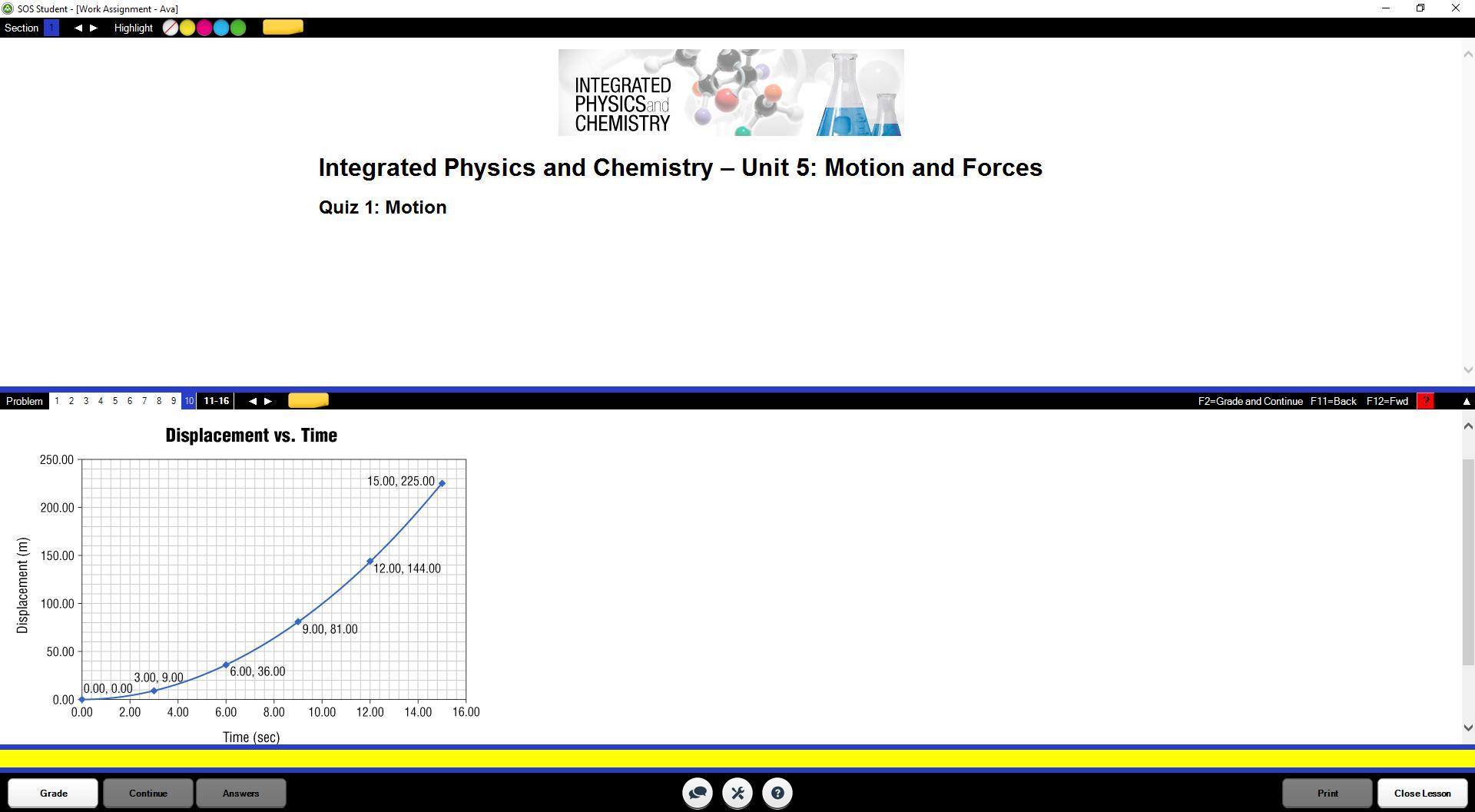
Answers
write a balanced chemical equation for the standard formation reaction of solid water .
Answers
The balanced chemical equation for the standard formation reaction of solid water is: H₂ (g) + 1/2O(g) → H₂O(s)
The standard formation reaction of solid water is: H₂ (g) + 1/2O₂(g) --> H₂O(s)
The balanced chemical equation for the standard formation reaction of solid water is: H₂ (g) + 1/2O(g) → H₂O(s)
The standard enthalpy change of the above reaction can be determined from the enthalpies of formation of the products and reactants using Hess's Law.
Standard enthalpy of formation (∆Hfo) is defined as the amount of heat absorbed or released when one mole of a substance is formed from its constituent elements in their standard states under standard conditions (∆Hfo=0 at 298 K and 1 atm).
Since H₂O(s) is the standard state of water, its standard enthalpy of formation (∆Hfo) is -285.8 kJ/mol. This means that the formation of one mole of solid water releases 285.8 kJ of heat energy.
Learn more about the Hess's Law from the given link-
https://brainly.com/question/16795968
#SPJ11
How is the Nernst equation used to find cell potential in concentration cells

Answers
The Nernst equation is used to find cell potential in concentration cells because the reaction quotient is used to find the actual cell potential, which is in option D. The Nernst equation is used to calculate the cell potential of an electrochemical cell when the reactants or products are not present in standard conditions, that is, when their concentrations or partial pressures are not 1 M or 1 atm, respectively.
The Nernst equation , E = E° - (RT/nF)lnQ
where E is the actual cell potential, E° is the standard cell potential, R is the gas constant, T is the temperature in Kelvin, n is the number of electrons transferred in the cell reaction, F is the Faraday constant, and Q is the reaction quotient.
In a concentration cell, both half-cells contain the same species but at different concentrations. Therefore, the reaction quotient is the ratio of the concentrations of the species in the two half-cells:
Q = [reactant] in cell 2 / [reactant] in cell 1.
Learn more about the Nernst equation here.
https://brainly.com/question/31747319
#SPJ1
Determine the equilibrium constant at 255 K for the following reaction under acidic conditions 4H+(aq) + MnO, (s) +2Fe2+ (aq)-> Mn2+ (aq) +2Fe3+ (aq) + 2H,0? The two half-reactions are: MnO2(s) +4H+(aq) + 2e-? Mn2+(aq) + 2H20(1) E?-1.23 V Fe3-(aq) + e-? Fe2 + (ag) E -0.770 V
Answers
The equilibrium constant (K) for the reaction at 255K is given by the Nernst equation.
What is equilibrium constant?
The value of a chemical reaction's reaction ratio at chemical equilibrium, a government that a dynamic chemical system approaches after enough time has passed and at which its structure has no discernible tendency to change further, is the equilibrium constant for that reaction. The equilibrium constant seems to be dependent on the initial analysis concentration levels of the product and reactant species in the mixture for a particular set of reaction conditions. As a result, the composition of a system at equilibrium can be calculated from its initial composition using known equilibrium constant values. However, factors affecting the reaction such as temperature, solvent, as well as ionic strength may all affect the equilibrium constant's value.
The equilibrium constant (K) for the reaction at 255K is given by the Nernst equation:
K = e^(-ΔG/RT)
where ΔG is the standard Gibbs free energy of reaction and R is the gas constant.
Using the half-reactions, we can calculate the standard Gibbs free energy of reaction (ΔG) as follows:
ΔG = -nF(E1-E2)
where n is the number of moles of electrons transferred in the reaction and F is the Faraday constant.
For the reaction above, n=2 and F=96,485 C/mol. Therefore,
ΔG = -2*96,485*(1.23-0.77) = -189.4 kJ/mol
Therefore, the equilibrium constant (K) at 255K is given by:
K = e^(-189.4/8.314*255) = 2.13 x 10^-11
To learn more about equilibrium constant
https://brainly.com/question/19340344
#SPJ4
a new amino acid is linked to the growing peptide chain. in order
Answers
A new amino acid is added to the growing peptide chain in a sequential order.
During protein synthesis, a new amino acid is added to the growing peptide chain in a specific and sequential order. This process occurs on the ribosomes, where transfer RNA (tRNA) molecules carry the corresponding amino acids and match them with the codons on the messenger RNA (mRNA) template.
The addition of a new amino acid involves several steps. First, an aminoacyl-tRNA synthetase enzyme attaches the appropriate amino acid to its corresponding tRNA molecule, forming an aminoacyl-tRNA complex. Then, this complex binds to the codon on the mRNA through complementary base pairing, ensuring the correct amino acid is positioned in the growing peptide chain.
With each cycle of protein synthesis, the ribosome advances along the mRNA molecule, and a new amino acid is added to the growing peptide chain. This sequential addition of amino acids follows the specific genetic code determined by the mRNA sequence, which ultimately determines the primary structure and identity of the protein being synthesized.
Overall, the process of adding a new amino acid to the growing peptide chain occurs in a precise order dictated by the mRNA sequence and is facilitated by the complementary base pairing between tRNA and mRNA codons.
To learn more about amino acid, here
https://brainly.com/question/31872499
#SPJ4
What properties of a soccer ball would change when it is filled with air particles?
Color and weight
Length and width
Size and shape
Texture and mass
Answers
which item is dull.
A. a glass cup
B. a cloth sock
C. a ceramic coffee mug
D. a metal pot
Answers
What vale is represented by the symbol Mr
Answers
the answer for this question is Mister
All of the following are qualitative tests that are often used to identify unknown ionic compounds except __________
Answers
All of the following are qualitative tests that are often used to identify unknown ionic compounds except titration.
Qualitative tests are conducted to determine the presence or absence of certain ions or compounds in a sample based on their characteristic reactions. Common qualitative tests include flame tests, precipitation reactions, color changes, and gas evolution tests.
Titration, on the other hand, is a quantitative technique used to determine the concentration of a specific substance in a solution by reacting it with a standardized solution of another substance. It is not typically used as a qualitative test for identifying unknown ionic compounds.
learn more about ionic compounds here
https://brainly.com/question/30420333
#SPJ11
the molar enthalpy of solution of a salt that dissolves endothermically in water is measured in a coffee-cup calorimeter by weighing a known amount of distilled water into the cup and measuring its temperature, then adding a known mass of the salt to the water and measuring the temperature after the salt dissolves. if some water is initially present in the cup before the weighed amount of water is added, what is the effect on the experiment?
Answers
Answer:
Explanation:
The molar enthalpy of solution of a salt that dissolves endothermically in water is measured in a coffee-cup calorimeter by weighing a known amount of distilled.
The boiling of the solution increases from that of solvent and this effect is called elevation of boiling point. It is a colligative property.
What is calorimetry?Calorimetry is an analytical tool used to measure the heat energy absorbed or evolved from a reaction. The reaction which absorbs energy is called an endothermic reaction.
The reaction which evolves heat energy is called exothermic reaction. The boiling of a substance is an endothermic process. When a non-volatile solute is added to a solvent then, the boiling point of the solvent increases from that of pure solvent.
It is a colligative property thus depends on the amount of solvent and solute particles. Here, the addition salt solution gives higher molar enthalpy than that of water.
To find more on calorimetry, refer here:
https://brainly.com/question/11477213
#SPJ2
Which of the following is an example of a chemical property?
o corrosion
o boiling point
o ability to dissolve
o melting point
Answers
Answer:
Corrosion is your answer
Answer:
▷The answer would be
▶︎corrosion
Explanation:
~corrosion~ is a natural process that converts a refined metal into a more chemically stable form such as oxide, hydroxide, or sulfide. It is the gradual destruction of materials by chemical and/or electrochemical reaction with their environment
✦plz mark me as brainliest☑︎
✭i only need 3 more brainliest then my lvl will go upp
what is metallic lustre please answer it as soon as possible I will make you brainlist
Answers
Metallic luster is a characteristic of metals in a compact state.
Answer:
It is a luster characteristic of metals in a state of compact and is also shown by other substances
Explanation:
Hope this helps. :)
4) The principle of ________ states that the physical, chemical, and biological processes at work shaping the Earth today have also operated in the geologic past.
A) catastrophism
B) plate tectonics
C) plutonism
D) Uniformitarianism
Answers
The principle of option D. Uniformitarianism states that the physical, chemical, and biological processes at work shaping the Earth today have also operated in the
Option D. Uniformitarianism is the principle stating that the physical, chemical, and biological processes at work shaping the Earth today have also operated in the geologic past. It is based on the idea that the present is the key to the past. In other words, the same natural laws that operate in the universe today have been operating since the beginning of time.
James Hutton was the first to propose this principle in the late 18th century. He suggested that the Earth was shaped by slow-acting geological forces such as erosion, sedimentation, and uplift over long periods of time. He believed that the same processes were still happening today and that they had operated in the past.
This principle is an important concept in geology because it allows scientists to interpret the Earth's history based on the processes that they observe today. By understanding how these processes work and how they have changed over time, scientists can reconstruct the history of the Earth and its environments.
Uniformitarianism has been tested and proven through many observations and experiments. For example, the study of sedimentary rocks has shown that they were formed in the past through the same processes that are observed today, such as deposition of sediment by water, wind, or ice.
Similarly, the study of volcanoes has shown that they are formed by the same processes as today, such as the movement of magma from deep within the Earth.
In conclusion, Uniformitarianism is the principle that allows us to interpret the Earth's history by observing the processes that shape it today. It is a fundamental concept in geology and has been tested and proven through many observations and experiments.
To know more about Uniformitarianism here
https://brainly.com/question/1324266
#SPJ11
Determine the oxidation number of chromium in potassium dichromate, K2Cr2O7.
Answers
Answer:
Explanation:
Consider the unbalanced equation for the oxidation of butene. C4H8 + 6O2 Right arrow. CO2 + H2O For each molecule of C4H8 that reacts, how many molecules of carbon dioxide and water are produced? Group of answer choices 2 CO2 & 2 H2O 4 CO2 & 4 H2O 2 CO2 & 4 H2O 4 CO2 & 2 H2O
Answers
450 J of heat are added to a 50 g block of lead. What is the resulting
temperature change? c for lead is 0.13 J/g(degC)
Answers
Answer:
69.2°C
Explanation:
Recall the heat energy formula
q = cmΔT
where q = heat energy ( measured in joules ) , c = specific heat , m = mass and ΔT = change in temperature.
We are given that:
The heat energy is 450J The mass is 50g The specific heat is 0.13 J/g(degC)Given this we want to find the temperature change.
First we want to define our variables.
Again recall that heat energy (q) = 450J , mass(m) = 50g and specific heat (c) = 0.13 J/g(degC)
Now that we have defined our variables we plug in the values of the variables into q = cmΔT and solve for the undefined variable
q = cmΔT
q = 450 , m = 50, c = 0.13
450 = (50) x (0.13) x (ΔT)
multiply 50 and .13
450 = 6.5 x (ΔT)
divide both sides by 6.5
69.2 = ΔT
The change in temperature was 69.2°C
Complete and balance the following equation K+H2O
Answers
Explanation:
hello
2K + 2H2O ==》2KOH + H2
pls helppp if possible

Answers
The new volume of an ideal gas is 5935.7 L. The volume of 23g neon is 1139.01 L. The pressure in Torr is 26537 T. Temperature in Celsius is 3483°C. The volume of the balloon will be 1121.7 L.
An ideal gas is a type of gas in which molecules do not attract or repel each other. They only interact through elastic collision. An ideal gas equation is the equation for ideal gas molecules.
Mathematically it is represented as :
PV = nRT
where:
P = Pressure in atm.
V = Volume in atm.
n = Number of moles.
R = Universal Gas Constant.
T = Temperature in Kelvin.
Given:
5. T = 100°C ( Converting into Kelvin : 100 + 273 = 373 K)
P = 1.4 atm
By substituting the values in the above-given equation:
V = 1 × 8.314 × 373 ÷ 1.4 = 5935.7 L
6. P = 2 atm.
T = 1° C (Converting Celcius into Kelvin: 273 + 1 = 274 K)
Given mass of Ne = 23g
The molar mass of Neon = 20g
Number of moles of Neon = Given mass ÷ Molar mass
23 ÷ 20 = 1.15 moles.
Substituting the values in the above-given equation:
V = 1.15 × 8.314 × 274 ÷ 2 = 1139.01 L
7. n = 11 moles.
V = 15 L.
T = 300°C ( Converting Celsius into Kelvin: 300+273 = 573 K)
Substituting values in the above given equation:
P = 11 × 573 × 8.31 ÷ 15 = 3491.8 atm
3491.8 atm into Torr = 26537 Torr
8. P = 6.5 atm.
V = 9.3 L.
n = 2.3 moles.
Substituting the values in the given equation:
T = 6.5 × 9.3 ÷ 2.3 × 8.314 = 218 Kelvin
218 Kelvin into Celsius is 55°C.
9. V₁ = 600mL.
P₁ = 700 mm
T₁ = 24° Celsius ( Converting Celsius into Kelvin: 24+273 = 297 K)
P₂ = 400 mm
T₂ = 5° Celsius ( Converting Celsius into Kelvin: 273+5 = 278K)
Substituting the values in the equation:
P₁V₁T₁ = P₂V₂T₂
V₂ = 700 × 600 × 297 ÷ 400 × 278 = 1121.7 L.
Learn more about, the ideal gas equation:
https://brainly.com/question/15379358
#SPJ1
Be as explicit as you can in describing how the covalent bond between an atom of Chlorine and an atom of Iodine forms. (What happens to the electrons, which electrons are involved, and what allows this to happen MUST all be part of your answer to receive full credit.)
Answers
When two atoms of chlorine and iodine join together to form a covalent bond, their outermost electrons interact to form a chemical bond.
What is a Covalent bond?
A covalent bond is a type of chemical bond that involves the sharing of a pair of electrons between two atoms. Covalent bonds are formed when two atoms come together to share their electrons in order to reach a more stable electron configuration. As a result, the two atoms become bonded together by the attraction of their shared electrons.
The chlorine atom has seven valence electrons, while the iodine atom has seven as well. Both atoms will want to complete their octets, so the two atoms share their outermost electrons with each other. During the formation of the covalent bond, the chlorine atom will donate one of its electrons to the iodine atom, while the iodine atom will donate one of its electrons to the chlorine atom. This sharing of electrons allows the two atoms to form a single bond and fill their octets, forming a covalent bond.
To know more about a Covalent bond,
https://brainly.com/question/3447218
#SPJ4
are protons attracted to neutrons or electrons or both?
Answers
Answer:
protons and electrons attract each other.
Explanation:
neutron as no charge, proton and electron are exactly the same size but opposite. ( the opposite charge attract)
The protons possess a positive charge so they attract negatively charged electrons.
What is a proton?A proton can be described as a stable subatomic particle, a symbol with a positive charge of +1e elementary charge. Proton's mass is slightly less than that of a neutron but 1836 times the mass of an electron.
Protons and neutrons are jointly referred to as "nucleons" each with masses of approximately one atomic mass unit. More than one proton can be present in the nucleus of an atom. They give the attractive electrostatic central force which holds the atomic electrons.
The number of protons in the nucleus is referred to as the atomic number and is represented by the symbol Z. Since each element in the modern periodic table has a unique number of protons, its own unique atomic number, determines the chemical characteristics of the element.
As the neutrons are neutral do not carry any charge so the protons do not attract neutrons.
Learn more about protons, here:
https://brainly.com/question/12535427
#SPJ2
Calculate the concentration of OH - ions in a 1.4 x 10-3 M HCl solution.Calculate the pH of the buffer system made up of 0.15 M NH3 and 0.35 M NH4Cl.
Answers
The concentration of OH⁻ ions in the HCl solution is 7.14 x 10⁻¹² M, and the pH of the buffer system is 8.88.
To calculate the concentration of OH⁻ ions in a 1.4 x 10⁻³ M HCl solution, follow these steps:
1. Determine the concentration of H⁺ ions: Since HCl is a strong acid, it completely dissociates in water, so the concentration of H⁺ ions is equal to the concentration of HCl, which is 1.4 x 10⁻³ M.
2. Use the ion product of water (Kw): Kw = [H⁺][OH⁻] = 1.0 x 10⁻¹⁴ at 25°C.
3. Solve for [OH⁻]: [OH⁻] = Kw / [H⁺] = (1.0 x 10⁻¹⁴) / (1.4 x 10⁻³) = 7.14 x 10⁻¹² M.
Now, to calculate the pH of the buffer system made up of 0.15 M NH₃ and 0.35 M NH₄Cl:
1. Determine the pKa of the weak acid (NH₄⁺): The acid dissociation constant, Ka, for NH₄⁺ is 5.56 x 10⁻¹⁰. pKa = -log(Ka) = 9.25.
2. Use the Henderson-Hasselbalch equation: pH = pKa + log([A⁻]/[HA]), where [A⁻] is the concentration of the weak base (NH₃) and [HA] is the concentration of the weak acid (NH₄⁺).
3. Plug in the values: pH = 9.25 + log(0.15 / 0.35) = 9.25 + log(0.4286) = 9.25 - 0.37 = 8.88.
Learn more about buffer system here:-
https://brainly.com/question/22821585
#SPJ11
The noble gas thought to be significantly carcinogenic due to its radioactive decay and that of its decay products is.
Answers
The answer is radon. Radon is a colorless and odorless gas that is formed naturally from the decay of uranium and thorium in soil, rock, and water. Radon is considered significantly carcinogenic because it emits alpha particles, which can damage the DNA in our cells and lead to cancer.
When inhaled, radon and its decay products can cause lung cancer, especially in people who are exposed to high levels over a long period of time.
In terms of its radioactivity, radon has a half-life of 3.8 days, which means that half of a given amount of radon will decay in that time. However, its decay products, such as polonium-218 and lead-214, also emit alpha particles and have longer half-lives. These decay products can attach to dust and other airborne particles, which can be inhaled and increase the risk of lung cancer.
In summary, radon is the noble gas that is significantly carcinogenic due to its radioactive decay and that of its decay products. It is important to test for radon levels in homes and workplaces and to take steps to reduce exposure if levels are found to be high.
To know more about DNA visit:
brainly.com/question/264225
#SPJ11