Why would you need to heat the hydrate up to 250 degrees if water boils at 100 degrees Celsius? Do you think that the boiling point of water in a hydrate might be different then pure water? Why?
Answers
The need to heat the hydrate up to 250 degrees is attributed to the water molecules that bond to the compound in a specific ratio known as the hydrate's formula. While it is true that water boils at 100 degrees Celsius, the boiling point of water in a hydrate may vary due to the presence of other molecules.
Furthermore, the hydration process is driven by the reaction between a compound and water molecules, which bond to form a specific number of hydrate molecules. When a hydrate is formed, a specific ratio of water molecules bonds to the compound. This bonding is through a process known as hydration, which results in a stable compound that can exist as a solid at room temperature.
Water molecules bond to the compound via hydrogen bonding, which involves the sharing of electrons. In this way, each molecule of the hydrate contains a fixed number of water molecules that can be removed by heating the compound. When the hydrate is heated, the water molecules evaporate, leaving behind the anhydrous compound. For instance, heating the hydrate Copper (II) sulfate pentahydrate, which has five molecules of water attached to one molecule of Copper sulfate, will release the water molecules and leave behind the anhydrous compound Copper (II) sulfate. This process is called dehydration. Therefore, the need to heat the hydrate to 250 degrees is because water molecules are bonded to the compound in a specific ratio that can only be removed by heating. While pure water boils at 100 degrees Celsius, the boiling point of water in a hydrate may vary due to the presence of other molecules in the compound.
To know more about molecules visit:
https://brainly.com/question/32298217
#SPJ11
Related Questions
How the calculation of the [OH-], pH and % ionization for 0.619 M ammonia (NH3) NH3 + H2O (liq) rightwards harpoon over leftwards harpoon NH4+ + OH- Kb = 1.8 x 10-5
Answers
Answer:
[OH⁻] = 3.34x10⁻³M; Percent ionization = 0.54%; pH = 11.52
Explanation:
Kb of the reaction:
NH3 + H2O(l) ⇄ NH4+ + OH-
Is:
Kb = 1.8x10⁻⁵ = [NH₄⁺] [OH⁻] / [NH₃]
As all NH₄⁺ and OH⁻ comes from the same source we can write:
[NH₄⁺] = [OH⁻] = X
And as [NH₃] = 0.619M
1.8x10⁻⁵ = [X] [X] / [0.619M]
1.11x10⁻⁵ = X²
3.34x10⁻³ = X = [NH₄⁺] = [OH⁻]
[OH⁻] = 3.34x10⁻³M% ionization:
[NH₄⁺] / [NH₃] * 100 = 3.34x10⁻³M / 0.619M * 100 = 0.54%
pH:
As pOH = -log [OH-]
pOH = 2.48
pH = 14 - pOH
pH = 11.52Calculate: The molecular mass of a molecule is the sum of the masses of each atom in the molecule. The
unit of molecular mass is the universal mass unit (u).
Iron's atomic mass is 55.85 u, carbon's mass is 12.01 u, and oxygen's mass is 16.00 u.
Calculate the molecular mass of carbon dioxide (CO2)
Answers
Answer:
44.01
Explanation:
12.01 + 16.00 + 16.00 = 44.01
What are the hydronium and hydroxide ion concentrations in a solution whose ph is 6.52?
Answers
A solution with a pH of 6.52 has a hydronium ion concentration of 3.02x10-7 mol/L and a hydroxide ion concentration of 3.31x10-8 mol/L.
The hydronium ion concentration of a solution can be calculated from pH by using \(10^{-pH}\). For a pH of 6.52, hydronium ion concentration is 3.02x10-7 mol/L.
The concentration of hydroxide ions can be determined by identifying the value of pOH. The sum of pOH and pH is equal to 14, which is based on the negative logarithm of the ion-product constant of water. At a pH of 6.52, pOH is equal to 7.48.
The relationship between pOH and hydroxide ion concentration is the same as the relationship between pH and hydronium ion concentration. With this, the hydroxide ion concentration at pOH of 7.48 is \(10^{-7.48}\) or 3.31x10-8 mol/L.
For more information regarding pH and pOH, please refer to the link https://brainly.com/question/13557815.
#SPJ4
what mass of sulfurous acid is produced when 245g of sulfur dioxide is reacted with water?
H₂O + SO₃ = H₂SO₃
Answers
The mass of sulfurous acid produced when 245g of sulfur dioxide is reacted with water is 313.8 g
StoichiometryFrom the question, we are to determine the mass of sulfurous acid produced
First, we will write the balaced chemical equation for the reaction
H₂O + SO₂ → H₂SO₃
This means 1 mole of H₂O reacts with 1 mole of sulfur dioxide (SO₂) to produce 1 mole of sulfurous acid (H₂SO₃)
Now, we will determine the number of moles of sulfur dioxide present
Mass of sulfur dioxide present = 245 g
Molar mass of sulfur dioxide = 64.066 g/mol
Using the formula,
\(Number \ of \ moles = \frac{Mass}{Molar\ mass}\)
Then,
Number of moles of SO₂ present = \(\frac{245}{64.066}\)
Number of moles of SO₂ present = 3.824 moles
This is the number of moles of SO₂ present
From balanced chemical equation
1 mole of H₂O reacts with 1 mole of sulfur dioxide (SO₂) to produce 1 mole of sulfurous acid (H₂SO₃)
Then,
Water (H₂O) will react with the 3.824 moles of sulfur dioxide (SO₂) to produce 3.824 moles of sulfurous acid (H₂SO₃)
∴ The number of moles of sulfurous acid produced is 3.824 moles
Now, for the mass of sulfurous acid produced
Molar mass of sulfurous acid = 82.07 g/mole
Using the formula,
Mass = Number of moles × Molar mass
Mass of sulfurous acid produced = 3.824 × 82.07
Mass of sulfurous acid produced = 313.8 g
Hence, the mass of sulfurous acid produced is 313.8 g
Learn more on Stoichiometry here: https://brainly.com/question/6061451
What happened to the solid lithium when the temperature was decreased?
A. Nothing changed.
B. Its shape changed.
C. Its volume changed.
Answers
The chemical element lithium has the symbol Li and atomic number 3. A delicate, silvery-white alkali metal, it is. It is the least dense solid element and the least dense metal under typical conditions.
What happens to the solid lithium when the temperature was decreased?
"Nothing changed."
With their high energy and power densities, lithium-ion batteries (LIBs) perform well in a variety of applications. However, the effect of temperature still has an impact on LIB performance. Normal operating range for LIBs is between 20 °C and 60 °C. Outside of this range, both low and high temperatures will result in decreased performance and permanent harm, such as thermal runaway and lithium plating.
At low temperatures, the components of a lithium-ion battery are also less compatible. The "vulnerability" of lithium-ion batteries at low temperatures is due to this. If a lithium-ion battery is overworked (high current charging and discharging), the resistance will increase and the capacity will decrease even more quickly. A "cold" lithium-ion battery will operate with greater resistance (increased resistance) and less effectively (rapid reduction in actual capacity).
To know more about Lithium, click on the link below:
https://brainly.com/question/2088293
#SPJ9
Balance the following equations. Enter 1 as a coefficient for reactants and products which have no coefficient.
___(NH4)2Cr2O7(s)➡️__Cr2O3(s) +___N2(g)+__H2O(g)
CO2(g) +__H20(l)__C6H12O6(s) +__C2(g)
Answers
1(NH4)2Cr2O7(s) → 1Cr2O3(s) + 1N2(g) + 4H2O(g).
6CO2(g) + 6H2O(i) → 1C6H12O6(s) + 6C2(g).
What types of reactants and the products are examples?Energy (the candlewick & wax) & oxygen are the reactants in the burning of a candle (in the air).Carbon dioxide and water vapor are the end results.
What distinguishes reactants from products?When K is bigger than 1, the reaction's products are preferred.The reactants inside the reaction were favored if K is much less than 1.When K is 1, neither the reactants nor the products are preferred.
To know more about coefficient for reactants visit:
https://brainly.com/question/3347921
#SPJ4
What units are used to measure mass and weight?
A. Mass and weight are measured in kilograms.
B. Mass and weight are measured in newtons.
C. Mass iş measured in kilograms, and weight is measured in newtons.
D. Mass is measured in newtons, and weight is measured in kilograms.
Answers
Answer:
the answer is A, they are measured in kilograms
Directions: Determine the molar mass of the following substances.
6. Nas
1. CaO
7. AICI:
2. Mg(CO3)
8. (14H4)2(SO4)
3. Zn(NO3)2
9. Ni3(PO4)2
4. Cuz(CrO4)
10. Ba(NnO4)2
5. Na(C103)
Answers
1. 40.08 + 16 = 56.08 g/mol CaO
2. 24.30 + 12.01 + 48.00 = 84.31 g/mol Mg(CO3)
3. 65.39 + 28.02 + 96 = 189.41 g/mol Zn(NO3)2
4. "Cuz" isn't an actual element. 52.00 + 64.00 = 116.00 g/mol CrO4
5. 22.99 + 1237.03 = 1260.02 g/mol Na(C103)
6. 22.99 + 32.06 = 55.05 g/mol NaS
7. 26.98 + 35.45 = 62.43 g/mol AlCl
8. 113.12 + 32.06 + 64 = 209.18 g/mol (14H4)2(SO4)
9. 176.07 + 61.94 + 128.00 = 366.01 g/mol Ni3(PO4)2
10. 137.33 + "Nn" isn't an actual element. 128.00 = 265.33 g/mol Ba(O4)2
When 10 moles HCl reacts with Ca(OH) 2 how many moles of H_{2}*O are made?
Answers
The amount of Ca(OH)₂ produced = 5.2 g which is calculated in the below section.
NUMBER OF MOLES of HCl = Molarity of solution x Volume of Solution
# of moles of HCl = (0.40 mol/L ) x 350 mL
= (0.40 mol/L ) x 0.350 L
= 0.14 mol
The mass of HCl that makes 0.14 mol of HCl
Mass of HCl= # of moles x molar mass of HCl
Mass of HCl = 0.14 mol x 36.5 g/ mol
Mass of HCl = 5.11g
As per Stoichiometry , 1g of HCl reacts with 1.015 g of Ca (OH)₂
So, 5.11g of HCl can react with 5.11 x 1.015 g
= 5.1865 g or 5.2 g of Ca(OH)₂
To learn more about number of moles check the link below-
https://brainly.com/question/29367909
#SPJ4
nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia:
Answers
Ammonia is created in the first step by the reaction of nitrogen and hydrogen.Nitric oxide & water are produced in the second step by the reaction of ammonia and oxygen."4NH (3)(g) + 5O (2)(g) to 6H (2)O(g) + 4NO" is a chemical formula.
How then can ammonia be changed into nitric acid and no2?I Reddish brown NO2 vapours are produced when ammonia is heated to 1073 K with oxygen and the addition of platinum gauze.Nitric acid is produced as a result of NO2 dissolving in water.As a result, ammonia is used to make HNO3.
What occurs when nitric acid and ammonia interact?Ammonium nitrate is created when nitric acid and ammonia combine (NH4NO3)
To know more about nitric oxide visit:
https://brainly.com/question/29357729
#SPJ4
what is the ionic charge lf this atom
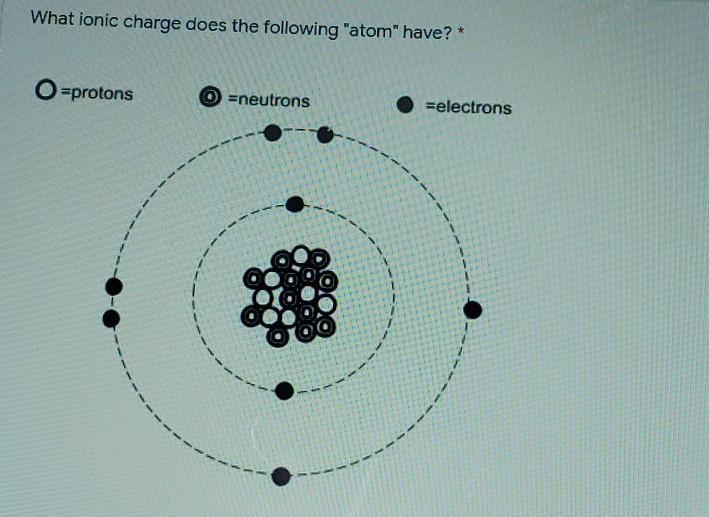
Answers
Answer:
An ionic charge of 2-
Explanation:
The number of electrons is 8 and the number of protons is 2, subtract the two and the difference is the ionic charge. Hope this helps! :)
Answer:
This answer would be 2
Explanation:
The way that I have always done it is you put protons in tally marks (without crossing) over the top of the neutrons and mark them out one by one and whichever one that you have leftover that is your charge.
Hope this helps!
g choose the arrow that most closely describes each question. the absorption with the lowest energy?
Answers
The arrow that most closely describes the question "the absorption with the lowest energy" is a downward-pointing arrow ↓.
In spectroscopy, particularly in electronic transitions, absorption refers to the process where a molecule or atom absorbs electromagnetic radiation, typically in the form of photons, causing the promotion of an electron from a lower energy level to a higher energy level. The energy difference between the two levels determines the energy of the absorbed photon.
When considering the absorption with the lowest energy, it implies that the absorbed photons have the lowest energy among the available energy levels. In this context, the downward-pointing arrow (↓) is used to represent the absorption of lower energy photons.
In spectroscopic diagrams or energy level diagrams, the upward-pointing arrow (↑) is typically used to represent the absorption of higher energy photons. However, since the question specifically asks for the absorption with the lowest energy, the appropriate arrow would be a downward-pointing arrow (↓).
Therefore, the arrow that most closely describes the question "the absorption with the lowest energy" is a downward-pointing arrow ↓.
Learn more about spectroscopy: https://brainly.com/question/28457917
#SPJ11
compared to the polar covalent bonds that hold the oxygen and hydrogen atoms together within a molecule of water, the hydrogen bonds that hold multiple water molecules together are much stronger.
Answers
The polar covalent bonds that hold the oxygen and hydrogen atoms together within a molecule of water, the hydrogen bonds that hold multiple water molecules together are much stronger.
The above statement is True.
Compared to the polar covalent bonds that hold oxygen and hydrogen atoms together within a water molecule, the hydrogen bonds that hold a together are much stronger. A hydrogen bond is a type of intermolecular force that occurs between a positively charged hydrogen atom of one molecule add a negatively charged oxygen or nitrogen atom of another molecule. These bonds are much stronger than the typical dipole-dipole forces that occur between polar molecules and are a major contributor to water's unique physical and biological properties.
Multiple water molecules are held together by strong, which are much more stronger than the polar covalent relationships that keep the hydrogen and oxygen atoms elements together within a single water molecule. As weak attractive forces, hydrogen bonds are much weaker than covalent polar bonds.
As a result, the proton end of the molecule is partly positively charged, whereas the oxygenation end is partially negatively charged. By virtue of its covalently bonded bonding and curving shape, water is characterized as a polar covalent. Molecular water hydrogen bonds Water molecules are attracted to each other happily because the their polarity.
A hydrogen atom that is slightly will bind to a hydrogen atom that is slightly in a hydrogen bond. They could be detected between water molecules.
Complete Question:
Compared to the polar covalent bonds that hold the oxygen and hydrogen atoms together within a molecule of water, the hydrogen bonds that hold multiple water molecules together are much stronger. True/False ?
To know more about Molecules click here
brainly.com/question/19922822
#SPJ4
What is the answer I need help I’m stuck

Answers
Answer:
Formation of covalent bonds in water
☆CHEERS!☆How many milligrams are equal to 0.0265 pounds?
(Dimensional Analysis)
Please show how you got that answer.
Answers
Answer:
37.73 pounds
Explanation:
just trust me.
na and k both use to passively diffuse across the plasma membrane. leak channels voltage-gated channels facilitated diffusion exocytosis carrier-mediated transport
Answers
Both Na and K use passive diffusion across the plasma membrane to leak channels.
The plasma membrane, also known as the cell membrane, is the membrane that separates the interior of a cell from its external environment. It may be found in all types of cells. A cell wall connects the plasma membrane to the outside of both bacterial and plant cells. The plasma membrane protects the cell from its surroundings while simultaneously controlling cellular mobility and sending signals. The plasma membrane, according to the fluid mosaic model, is a patchwork of phospholipids. Non-gated ion channels are ones that are always open. Because they simply allow ions to pass through the channel without causing any damage.
To learn more about diffusion please click on below link
https://brainly.com/question/13513898
#SPJ4
in the photosynthesis reaction, 6co2 + 6h2o → c6h12o6 + 6o2, there are 18 oxygen atoms in the reactants. how many oxygen atoms are in the products?
Answers
There are also 6 oxygen atoms in the products. This is because the products of the photosynthesis reaction include C6H12O6 (glucose) and 6O2 (oxygen gas). The glucose molecule contains 6 carbon, 12 hydrogen, and 6 oxygen atoms.
In the photosynthesis reaction, 6CO2 + 6H2O → C6H12O6 + 6O2, there are 18 oxygen atoms in the reactants.
Therefore, the total number of oxygen atoms in the products is 6. This is because oxygen gas is not bonded to anything else and is released into the atmosphere as a product of photosynthesis.
The reactants of photosynthesis, carbon dioxide (CO2) and water (H2O), are converted into glucose (C6H12O6) and oxygen (O2) gas. In this process, sunlight energy is converted into chemical energy that is stored in glucose, which is used by the plant for growth and other metabolic processes. Therefore, photosynthesis is a crucial process for life on earth.
To know more about photosynthesis visit:
https://brainly.com/question/30715543
#SPJ11
Help me pls!!
List all three sets of opposing forces.
Answers
Answer:
System-wide consistency versus institutional autonomy, efficient versus effective assessment, and promotion of student progression versus enforcement of academic standards.
which medium is denser between water and glass
Answers
What is the concentration, in M, if 227 grams of Na2SO4 are dissolved in 1.10
liters?
A 206 M
B 5.11 M
C 2.83 M
D 1.45 M
Answers
Answer:
D 1.45 M
Explanation:
Why do scientific experiments need to be repeated?
A. Because scientists often make mistakes
B. To ensure that the same results are achieved every time
C. They only need to be repeated if the results are questionable
D. To discourage other scientists from trying to get the same results
Answers
Answer:
B
Explanation:
this is called peer review and your results will be stronger
6. What type of relationship is shown in the image below?
1 point
500
400
300
Mass, 9
200
100
0
0
200
400
600
800
1000
Volume, ml
Direct
Exponential
O Indirect
Inverse

Answers
Answer:
A direct relationship
Explanation:
The graph shows a direct relationship due to the fact that the line is going up in a positive correlation/increasing as the amount of mass and volume increase.
When a drawing of a circle made in the center of a piece of paper with a black marker gets wet, the marker bleeds, and as the water moves through the paper the ink separates into several colors.
Answers
Answer:
Chromatography
Explanation:
Chromatography is a technique for separating mixtures that involves the use of a moving liquid and filter paper. The solvent travels through the spots on the paper, taking the chemicals away in the opposite direction. If the solvent combination has been properly chosen, each of them will move at a different velocity than the others.
What two of the following organisms are secondary consumers in this food web?
Answers
Secondary consumers are organisms that primarily feed on herbivores or other primary consumers.
They occupy the next trophic level above the primary consumers in a food web. They obtain energy by consuming the primary consumers and play an important role in regulating the population of herbivores.
Examples of commonly observed secondary consumers include:
Carnivorous mammals: Animals such as wolves, lions, and tigers that feed on herbivores like deer, zebras, or gazelles.
Birds of prey: Species like eagles, hawks, and owls that consume small mammals, reptiles, or other birds.
Carnivorous fish: Fish like pike, barracuda, or bass that prey on smaller fish or aquatic invertebrates.
Predatory insects: Insects such as spiders, mantises, or dragonflies that feed on other insects, including herbivorous insects.
In a specific food web, the identification of secondary consumers would depend on the specific organisms present and their feeding interactions. It would be necessary to analyze the trophic relationships among the organisms in the food web to determine the secondary consumers accurately.
For more such questions on Secondary consumers visit:
https://brainly.com/question/28631974
#SPJ8
I need help please help me

Answers
chemical potential energy, thermal energy, electrical energy, sound energy
Explanation:
the coal is chemical potential energy because it is just sitting there, the coal fueled power plant is thermal energy because it is using the heat from the coal to work the power plant, that is then turned into electrical energy, which then goes to the radio which displays sound energy
pls give brainliestWhat conditions will dissolve solute the fastest? (think about temperature, particles, etc.)
Answers
When you are dispensing stock solution into your graduated cylinder, you find that you have poured out too much solution. What is the best thing to do with the excess solution? a. use the whole amount in the experiment om. b. pour into the waste container. c. pour back into the stock bottle. d. pour down the sink drain
Answers
When you have poured out too much solution while dispensing a stock solution into a graduated cylinder, the best thing to do with the excess solution is to pour it back into the stock bottle.
Pouring the excess solution back into the stock bottle is the recommended course of action for several reasons. Firstly, it helps to maintain the accuracy and integrity of the stock solution. By returning the excess solution to the stock bottle, you ensure that the concentration of the solution remains as intended. This is important for future experiments or for other researchers who may use the same stock solution.
Secondly, pouring the excess solution into the waste container or down the sink drain can be wasteful and environmentally unfriendly. It is best to minimize waste and avoid unnecessary disposal of chemicals whenever possible.
Lastly, using the whole amount of the excess solution in the experiment may lead to inaccurate results or affect the desired concentration of the solution. It is important to carefully measure and control the amount of solution used in an experiment to ensure reliable and reproducible data.
Learn more about stock solution here:
https://brainly.com/question/28083950
#SPJ11
Chlorine has two naturally occurring isotopes, 35cl and 37cl. What is the mass number of each? how many protons, neutrons, and electrons are present in each?.
Answers
The mass number \(_{17} Cl^{35}\) is 35.
The mass number \(_{17} Cl^{37}\) is 37.
isotopes of \(_{17} Cl^{35}\) contains. and isotopes \(_{17} Cl^{37}\) contain.
protons=17 protons= 17
neutrons= 18 neutrons=20
electrons= 17 electrons=17
Define Isotopes
Isotopes are two or more atom types that share the same atomic number (number of protons in their nuclei) and position in the periodic table (and, therefore, belong to the same chemical element). However, isotopes have different nucleon numbers (mass numbers) because they have varying numbers of neutrons in their nuclei. All isotopes of a given element have nearly identical chemical properties but differ in terms of atomic mass and physical characteristics.
Learn more about Isotopes here:-
https://brainly.com/question/24337473
#SPJ4
remember that a single, double or triple bond counts as a single electron group. each lone pair also counts as a single electron group. if you know the number of electron groups then you can predict the hybridization. true or false
Answers
The statement "if you know the number of electron groups then you can predict the hybridization" is True.
This means that if you know the number of electron groups, then you can predict the hybridization. Let us understand what hybridization is.
Hybridization is a theory that explains the concept of the mixing of atomic orbitals to form new hybrid orbitals. These hybrid orbitals arrange themselves in a specific manner so that the valence electrons of the atoms involved can pair up with another atom to form a chemical bond.
Lone pair electrons and bond pair electrons are collectively referred to as electron groups. These groups are used to determine the shape and geometry of the molecule. A single, double, or triple bond counts as a single electron group, and each lone pair also counts as a single electron group. Hence, the total number of electron groups in a molecule is the sum of the number of bonding and lone pairs.
To predict hybridization, you need to count the total number of electron groups around the central atom. The number of hybrid orbitals required is the same as the number of electron groups. For example, if there are four electron groups around the central atom, the hybridization will be \(sp^3\).
Therefore the given statement is true.
To learn more about hybridization refer to: https://brainly.com/question/14140731
#SPJ11
.What is the final chemical equation from the following intermediate chemical equations?
P4O6(s) → P4(s) + 3O2(g)
P4(s) + 5O2(g) → P4O10(s)
Answers
To obtain the final chemical equation, we need to combine the given intermediate equations in a way that cancels out the common compounds and species.
The intermediate equations are:
P4O6(s) → P4(s) + 3O2(g)
P4(s) + 5O2(g) → P4O10(s)
To combine these equations, we can multiply equation 1 by 2 to balance the number of moles of P4:
2P4O6(s) → 2P4(s) + 6O2(g)
Now, we can add equation 2 to the modified equation 1 to cancel out the common compounds (P4 and O2) and obtain the final balanced chemical equation:
2P4O6(s) + P4(s) + 5O2(g) → 2P4(s) + 6O2(g) + P4O10(s)
Simplifying the equation, we have:
2P4O6(s) + P4(s) + 5O2(g) → 2P4O10(s)
Therefore, the final chemical equation from the given intermediate equations is:
2P4O6(s) + P4(s) + 5O2(g) → 2P4O10(s)
The final balanced chemical equation from the following intermediate chemical equations is 2P4O6(s) + P4(s) + 5O2(g) → 2P4O10(s).
To know more about compounds , visit:
https://brainly.com/question/14658388
#SPJ11