WILL AD BRAINLIST write 5 sentences you have learned from observing rock samples. make sure to include what you know about your sediment in your response. and include the environment of the past
Answers
Related Questions
a plant only requires the correct chemicals to make plant food ture or false
Answers
Answer:
Your correct answer is true.
Explanation:
A plant only requires the correct chemicals to make plant food. ( True )
a sample of alcohol has a density 0.82 g/ml.what is the mass of 5500 mL of the alcohol
Answers
Answer:
The answer is 4510 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
density = 0.82 g/mL
volume = 5500 mL
We have
mass = 0.82 × 5500
We have the final answer as
4510 gHope this helps you
A block of concrete has a mass of 5100 g and a volume of 2500 cm3 . Calculate the density.
Answers
Answer:
2.04 g/cm3
Explanation:
Density = mass ÷ volume
= 5100 ÷ 2500
=2.04 g/cm3
You now need to make 150 mL of a 1 mg/mL hemoglobin solution. How many mg will you need?
Your scale measures mass in grams. How many grams of hemoglobin will you need to make the above solution?
Answers
150mg, which is equivalent to 0.15g, will be needed to make 150mL of the hemoglobin solution.
How to convert units of mass?Mass is a quantity of matter which a body contains, irrespective of its bulk or volume. It is one of four fundamental properties of matter. It is measured in kilograms in the SI system of measurement or grams, milligrams etc.
According to this question, one needs to make 150 mL of a 1 mg/mL hemoglobin solution. This means that;
1mg = 1mL
Hence, 150mL will need 150mg
Since 1mg = 0.001g
150mg = 150 × 10-³ = 0.15grams.
Learn more about mass at: https://brainly.com/question/14953134
#SPJ1
Calculate the answer. Express It In scientific notation. All answers should have the correct number of significant figures.
(4.9 x 10^-2) (9.80 x 10^2) =
48.02
48
48 x 10^-1
4.8 x 10^1
Answers
Answer:
4.8 x 10^1
Explanation:
Move the decimal so there is one non-zero digit to the left of the decimal point. The number of decimal places you move will be the exponent on the 10. If the decimal is being moved t the right, exponent will be negative. If the decimal is being moved to the left, the exponent will be positive.
How can air quality be fine on one day and hazardous on the next? What
are some things that can cause a rapid change in air quality? *
Answers
Answer:
Air Current
Explanation:
Say I am in California and there is a massive fire next to me but not close enough to me to really see the fire and the next day I see no fire but theres a ton of ash in the sky, this can be due to the wind current taking the smoke from the fire into my direction and that smoke falling in my area. So basically a wind current can take the smoke from a fire into another destination and cause a rapid change in air quality in a day.
Holding your hand over a hot plate is an example of
because
Answers
Answer:
Radiation
Explanation:
explanation: idek
The poh of a solution is 10. 75. What is the concentration of oh– ions in the solution? use. 3. 162 10–108 m 1. 778 10–11 m 1. 075 102 m 5. 623 1010 m.
Answers
Based on the pOH of the solution, the hydroxide ion concentration, [OH-] of the solution is 1.778 × 10^-11 M
The pOH of a solution-
The pOH of a solution is the negative logarithm to base ten of the hydroxide ions concentration of the solution.
pOH = -log[OH-]
The pOH of the solution = 10.75
[OH-] = 10^(-pOH)
Hydroxide ion concentration, [OH-] = 10^-10.75
Hydroxide ion concentration, [OH-] = 1.778 × 10^-11 M
Therefore, the hydroxide ion concentration, [OH-] of the solution is 1.778 × 10^-11 M
Learn more about pOH at:
brainly.com/question/13557815
#SPJ4
If the nucleotidcompared to the shoulder, displacements of the hip joints are ________.
Answers
If the nucleotide compared to the shoulder, displacements of the hip joints are larger.
The comparison of the nucleotid to the shoulder can be used to understand the movement of the hip joints as well. As the shoulder extends downward, the hip can originate from a point of flexion before it extends up and outward.
This is a result of the vertical pull of the shoulder being countered by the equal and opposite force of the hip pulling in the opposite direction. The hip is able to take some of the load off the shoulder, allowing for a greater range of motion in the shoulder movement. With the hip helping to counter the shoulder movement, a larger range of motion is achieved.
When it comes to displacing the hip joints, it is important to understand the mechanics of the movement. Movement of the hip joint often begins with a slight posterior rotation of the pelvis which helps bring the femur back into a neutral position before it extends up and outward.
know more about nucleotide here
https://brainly.com/question/16308848#
#SPJ11
Answer the following questions:
1. Define a compound. State three points of evidence to show that sodium chloride is a compound.
2. When Iron powder and Sulphur powder were mixed together and heated strongly, a substance A was formed. When did. Hydrochloric acid was added to substance A, the gas B was evolved.
a) What is A and what type of substance is it?
b) Name the gas B. State one characteristic property of gas B.
c)Is the reaction of Iron and Sulphur powder a chemical change or a physical change.
Answer the question according to the class 9th student.
Answers
Answer:
1. A compound is a group of elements that cannot be separated by physical methods.
NaCl is a compound becuse it cannot be separated by physical methods.
It consists of postive and negative ions.
It can be separated only by chemical reactions.
2. (a) A is a mixture of iron and sulphur and it is in liq form.
(b) Gas B is hydrogen gas and it has sharp pungent irritating smell.
(c) It is a physical change.
Explanation:
Due to the number of requirements for a successful collision, according to the collision theory, the percentage of successful collisions is extremely small. yet, chemical reactions are still observed at room temperature and some at very reasonable rates. explain
Answers
According to the collision theory, successful collisions leading to chemical reactions are rare due to the numerous requirements. However, some reactions still occur at room temperature and at reasonable rates.
The collision theory states that for a chemical reaction to occur, molecules must collide with sufficient energy and with the correct orientation. Additionally, they need to overcome the activation energy barrier, which is the minimum energy required for a reaction to proceed. Considering these requirements, the percentage of successful collisions is actually quite small.
However, chemical reactions are still observed at room temperature and some even proceed at reasonable rates. This can be attributed to several factors. Firstly, although the probability of a successful collision is low, the vast number of molecules in a given sample increases the chances of collisions occurring.
Additionally, the presence of catalysts can lower the activation energy, facilitating the reaction and increasing the rate of successful collisions. Furthermore, the use of higher temperatures increases the kinetic energy of the molecules, making it more likely for them to possess the required energy for a successful collision.
Learn more about chemical reactions here:
https://brainly.com/question/29762834
#SPJ11
Which is one source of the sediments that form sedimentary rocks?
Answers
Answer:
Sandstone, limestone, and shale
Explanation:
The rocks are most likely to start as sediments carried in rivers and end up in lakes and oceans
Answer:
D - materials dissolved in solutions
Explanation:
EDGE 2021
which one of these molecules has a chiral center? which one of these molecules has a chiral center? ch3ch(cl)ch2ch3 ch2cl2 ch3c(cl)2ch2ch3 ch3ch2ch2ch3 none of these has a chiral center
Answers
The molecule that has a chiral center is ch3c(cl)2ch2ch3.
A chiral center, also known as a stereogenic center or an asymmetric carbon, is a carbon atom bonded to four different substituents.
In the given molecules, only ch3c(cl)2ch2ch3 satisfies this condition. The central carbon atom (denoted by the asterisk *) is bonded to a hydrogen atom (H), two methyl groups (CH3), and a chlorine atom (Cl), making it a chiral center. The presence of a chiral center allows for the molecule to exist in two enantiomeric forms, which are non-superimposable mirror images of each other. The other three molecules do not have a chiral center and, therefore, are not chiral.
In summary, out of the given molecules, only CH3C(Cl)2CH2CH3 has a chiral center.
Learn more about chiral center https://brainly.com/question/9522537
#SPJ11
Compounds are made up of different kinds of atoms that are chemically combined. What do compounds have in common? A. They have the same color. B. They have the same melting point. C. They can be broken down into simpler substances. D. They cannot combine with one another to form more complex substances.
Answers
Answer:
D
Explanation:
They cannot combine with one another to form more complex substances. that's the answer
The compounds have in common is they cannot combine with one another to form more complex substances. The correct option is D.
What are compounds?Compounds are the substances made up of numerous similar molecules made up of atoms from different elements and kept together by chemical bonds. Therefore, a molecule made up of only one type of atom is not a compound.
There are different types of compounds that are made by combining of different substances. Like, ionic compounds are made by combining metals and non-metals.
Compounds are the substance that can into form further complex substance by combining with another substance because they are already made up of two or more substance.
Thus, the correct option is D. They cannot combine with one another to form more complex substances.
To learn more about compounds, refer to the below link:
https://brainly.com/question/14658388
#SPJ2
The natural cements that hold clasts together precipitate in the empty pore spaces after compaction. Those precipitates come from ______. Multiple choice question. water containing dissolved materials the alignment of clay particles outward growth of the original clasts
Answers
This cementation process contributes to the consolidation and hardening of sedimentary rocks over time.
What is the source of natural cements that bind clasts together in sedimentary rocks?The natural cements that bind clasts together and precipitate in the void spaces after compaction originate from water containing dissolved materials.
As sediments undergo compaction due to the weight of overlying layers, the pore spaces between clasts become smaller.
Water present in these pores carries dissolved substances such as minerals and ions.
As the water is squeezed out during compaction, these dissolved materials are left behind and begin to precipitate.
The precipitation process involves the formation of mineral crystals that gradually fill the pore spaces, effectively cementing the clasts together.
Learn more about cementation process
brainly.com/question/30751708
#SPJ11
. Consider a half life of 5.3years for Co-60. Exactly 15.9 years ago you start with a Co-60 sample with an initial decay rate of 15uCi . What is the strength of the source now
Answers
The strength of the source now with sample with an initial decay rate of 15uCi is 711.
An unstable element is transformed into a more stable one by radioactive decay, which involves the loss of elementary particles from the unstable nucleus. Alpha emission, beta emission, positron emission, electron capture, and gamma emission are the five different kinds of radioactive decay.
Each sort of decay releases a distinct particle that modifies the kind of product created. The sort of decay or emission that the initial element experiences determines how many protons and neutrons are present in the daughter nuclei, which are the nuclei generated during the decay.
Half life = 0.693 / λ,
λ = disintegration constant
λ = 0.693 / (5.3 x 365 x 3600) = 9.95 x 10⁻⁸
2.65 years before decay rate = λN₀ = 9.95 x 10⁻⁸ x N₀
N₀ = 1005
\(N = N_0e^{-\lambda t}\)
= 1005x (2.65 x 365 x 3600) x \(e^{-9.95*10^-^8}\)
N = 710.7 = 711.
Therefore, strength of the source now is 711.
Learn more about Decay rate:
https://brainly.com/question/23499960
#SPJ4
if the same amount of heat is added to 50.0 g samples of each of the metals, which are all at the same temperature, which metal will reach the highest temperature?
Answers
The metal which will reach the highest temperature is the metal with the lowest specific heat capacity.
What is the amount of heat added to each metal?The amount of heat Q = mcΔT where
m = mass of metalc = specific heat capacity of mateal and ΔT = temperature changeTemperature change of the metalMaking ΔT subject of the formula, we have
ΔT = Q/mc
Given that Q and m are the same for each metal,
ΔT ∝ 1/c
We see that the temperature change is inversely proportional to the specific heat capacity.
Since the metals are at the same temperature, the metal which will reach the highest temperature is the metal with the lowest specific heat capacity.
So, the metal which will reach the highest temperature is the metal with the lowest specific heat capacity.
Learn more about temperature here:
https://brainly.com/question/16559442
#SPJ12
experiment 1: calculate the combined mass of the two reactants: hydrochloric acid and sodium hydroxide
Answers
The combined mass of hydrochloric acid and sodium hydroxide is determined by adding their individual masses.
When calculating the combined mass of hydrochloric acid and sodium hydroxide, we need to consider the individual masses of these two substances. Hydrochloric acid (HCl) has a molecular formula of HCl and consists of one hydrogen atom (H) and one chlorine atom (Cl). Sodium hydroxide (NaOH), on the other hand, is composed of one sodium atom (Na), one oxygen atom (O), and one hydrogen atom (H). To calculate the combined mass, we add the individual masses of these reactants.
The molar mass of hydrogen (H) is approximately 1 gram/mol, while the molar mass of chlorine (Cl) is approximately 35.5 grams/mol. Sodium (Na) has a molar mass of around 23 grams/mol, oxygen (O) has a molar mass of approximately 16 grams/mol, and hydrogen (H) has a molar mass of around 1 gram/mol.
To determine the combined mass of hydrochloric acid and sodium hydroxide, we multiply the number of atoms of each element by their respective molar masses and sum them up. For example, hydrochloric acid has one hydrogen atom and one chlorine atom, so the total mass would be 1 gram/mol (hydrogen) + 35.5 grams/mol (chlorine). Similarly, sodium hydroxide has one sodium atom, one oxygen atom, and one hydrogen atom, resulting in a combined mass of 23 grams/mol (sodium) + 16 grams/mol (oxygen) + 1 gram/mol (hydrogen).
Learn more about hydrochloric acid
https://brainly.com/question/1451933
#SPJ11
The d orbital starts in the 4th row, or 4th energy level. However, what energy level (period number) does d actually start with?
Answers
The d-orbital starts in the third energy level (n = 3) of an atom.
Each energy level can contain one or more sublevels, including s, p, d, and f sublevels. The first energy level (n = 1) has one s orbital and can hold a maximum of 2 electrons. The second energy level (n = 2) has one s orbital and three p orbitals, allowing for a maximum of 8 electrons. The third energy level (n = 3) has one s orbital, three p orbitals, and five d orbitals, accommodating a maximum of 18 electrons.
The d-orbitals are found in the third energy level, corresponding to the third period of the periodic table. Therefore, the period number for the energy level where the d-orbital starts is 3.
The filling order of orbitals follows the pattern: 1s, 2s, 2p, 3s, 3p, 4s, 3d, and so on. The d-orbitals start filling after the p-orbitals in the third energy level. The electron configuration for the third energy level is written as 3s^2 3p^6 3d^1-10, depending on the element.
For example, the electron configuration of iron (Fe) is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^6 4s^2. This configuration indicates that the d-orbitals of iron are half-filled with 5 electrons.
Learn more about energy level
https://brainly.com/question/30546209
#SPJ11
The mass of a sample of CaCO3is 751 grams. How many total atoms are present in this sample? Possible options: a) 4.51×1022 atoms b) 9.04x1024 atoms c) 2.26×1025 atoms d) 6.02x1026 atoms
Answers
To determine the total number of atoms present in a sample of CaCO3 with a mass of 751 grams, we need to calculate the number of moles of CaCO3 and then multiply it by Avogadro's number, which represents the number of atoms in one mole of a substance.
Given the molar mass of CaCO3, we can calculate the number of moles, and by multiplying it with Avogadro's number, we can determine the total number of atoms in the sample. The correct option among the given choices is (b) 9.04x1024 atoms.
The molar mass of CaCO3 can be calculated by adding the atomic masses of its constituent elements: Ca (calcium), C (carbon), and O (oxygen). The atomic masses are found on the periodic table.
CaCO3 = (1 mol Ca) + (1 mol C) + (3 mol O)
= (40.08 g/mol) + (12.01 g/mol) + (16.00 g/mol) * 3
≈ 100.09 g/mol
Next, we can calculate the number of moles of CaCO3 in the sample:
moles = mass / molar mass
= 751 g / 100.09 g/mol
≈ 7.50 mol
Now, to determine the total number of atoms, we multiply the number of moles by Avogadro's number:
total atoms = moles × Avogadro's number
= 7.50 mol × (6.02 × 10^23 atoms/mol)
≈ 4.51 × 10^24 atoms
Therefore, the correct option is (b) 9.04x1024 atoms, which is the closest approximation to the calculated value.
To learn more about total number of atoms visit: brainly.com/question/13073376
#SPJ11
The mass of a sample of CaCO3 is 751 grams. The number of total atoms in this sample is approximately 10^22 CaCO3 molecules.
Explanation:The molar mass for calcium carbonate (CaCO3) is computed to be 100.0869 g/mol. The given mass of the sample (751 g) is a little less than one-tenth the mass of 1 mole of CaCO3 (~10 g), so a reasonable estimate for the number of CaCO3 molecules in the sample would be on the order of one-tenth Avogadro's number (NA), or approximately 1022 CaCO3 molecules. Therefore, we can conclude that there are approximately 1022 CaCO3 molecules in the sample.
Learn more about Number of atoms in a sample here:https://brainly.com/question/32351065
#SPJ12
N2O4 ⇌ 2NO2
for the following reaction at 373 K, Kc = 0.36. If initial concentration of N2O4 is 0.1 mol dm^-3, what is the equilibrium concentration of NO2? (Is there a way to solve this without using quadratics?)
Answers
Okay, let's solve this step-by-step without using quadratics:
1) The equilibrium constant Kc = 0.36 means the equilibrium lies to the left. So there will be more N2O4 than NO2 at equilibrium.
2) The initial concentration of N2O4 is 0.1 mol dm^-3. Let's call this [N2O4]initial.
3) At equilibrium, the concentrations of N2O4 and NO2 will be [N2O4]equil and [NO2]equil respectively.
4) We know the equilibrium constant expression for this reaction is:
Kc = ([NO2]equil)^2 / [N2O4]equil
5) Setting this equal to 0.36 and plugging in 0.1 for [N2O4]initial, we get:
0.36 = ([NO2]equil)^2 / (0.1 - [NO2]equil)
6) Simplifying, we get:
0.036 = [NO2]equil^2
7) Taking the square root of both sides, we get:
[NO2]equil = 0.06 mol dm^-3
So the equilibrium concentration of NO2 is 0.06 mol dm^-3.
Let me know if you have any other questions! I can also provide a more step-by-step explanation if needed.
7. What is the name of: CH3-CH(CH3)-CH2- alky group? *
isopropyl
propyl
sec-butyl
butyl
Answers
Answer:
propyl
1-propylmethane or 2-methylpropane
Calculate the heat change associated
with cooling a 350.0 g aluminum bar
from 70.0/C to 25.0/C. Is the change
endothermic or exothermic? Why? How to change -14,175J to -14.2kj
(-14.2 kJ)
Answers
The aluminum bar is cooling through an exothermic process, which means that heat is being released from the bar and being transported to the surroundings. This is indicated by the negative sign of the heat change.
Exothermic or endothermic change?The aluminum bar is cooling through an exothermic process, which means that heat is being released from the bar and being transported to the surroundings. This is indicated by the negative sign of the heat change (-14.2 kJ). This is due to the metal bar losing heat as it cooled down because the ultimate temperature (25.0°C) is lower than the initial temperature (70.0°C).
we can use the formula:
Q = m * c * ΔT
where Q = heat change, m = mass of the aluminum bar, c = specific heat capacity of aluminum, and ΔT = change in temperature.
The specific heat capacity of aluminum is 0.903 J/g·°C.
So, plugging in the values, we get:
Q = (350.0 g) * (0.903 J/g·°C) * (25.0°C - 70.0°C)
Q = -14,175 J
The negative sign indicates that heat is being lost by the aluminum bar,
To convert -14,175 J to kJ, we divide by 1000:
-14,175 J = -14.175 kJ
Rounding to one decimal place, we get:
-14.175 J ≈ -14.2 kJ
To know more about the exothermic change visit:
https://brainly.com/question/30802821
#SPJ1
Drag and drop each of the three molecular sizes to the location each would occur during the process of column chromatography.
Answers
The correct positions of the different molecular sizes within the column chromatography are as follows: Small molecules: Start, Intermediate molecules: Middle, and Large molecules: End
Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each other while moving with the aid of a mobile phase.
During the process of column chromatography, the different molecular sizes will settle at different positions. The small molecules are going to travel through the column at a much slower pace than the larger molecules. The three molecular sizes involved in the process of column chromatography are the following:
i) Small molecules
ii) Intermediate molecules
iii) Large molecules
Now, we have to place these molecules in their respective positions. The following is a drag and drop table which shows the positions of each molecule within the chromatography process:
Molecule size Position
Small molecule Start
Intermediate molecule Middle
Large molecule End
Therefore, the correct positions of the different molecular sizes within the column chromatography are as follows:
Small molecules: Start Intermediate molecules: Middle Large molecules: End
To know more about chromatography, visit:
https://brainly.com/question/11960023
#SPJ11
the process of converting sensory data into electrical and chemical signals is called
Answers
Answer:
Sensory transductionThe main function of sensory transduction is the conversion of sensory signals to chemical signals in the nervous system. Sensory transduction occurs in the sensory receptors. In sensory transduction, the sensory neurons play an important role.
Explanation:
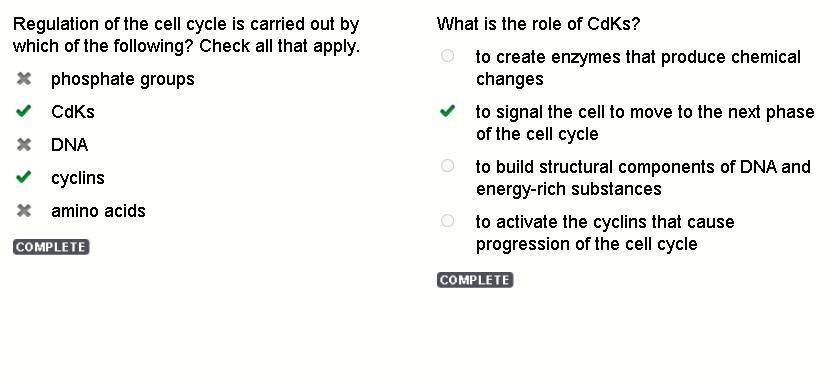
if it helped uh please mark me a brainliest :))What is the role of CdKs? to create enzymes that produce chemical changes to signal the cell to move to the next phase of the cell cycle to build structural components of DNA and energy-rich substances to activate the cyclins that cause progression of the cell cycle
Answers
Answer:
It’s b
Explanation:
Answer:
Regulation of the cell cycle is carried out by which of the following? Check all that apply.
CdKs
cyclins
What is the role of CdKs?
to signal the cell to move to the next phase of the cell cycle
Explanation:
Is starch an ionic or covalent compound?
Answers
Starch is a covalent compound as it possesses long chains of glucose molecules that are bonded through covalent bonds.
What are covalent compounds?In a covalent compound, the covalent bonds are involved where the mutual sharing of electrons that can hold all atoms or molecules together. Shared electrons are difficult to give away as nuclei of two atoms together share the electrons and create the bond stronger.
Ionic compounds can be demonstrated as compounds prepared between a cation and an anion. A cation can be defined as an electropositive ion and can lose valence electrons. Similarly, anions are electronegative ions and can a tendency to gain electrons.
Starch can be defined as a polysaccharide composed of 1,4 linkages between glucose monomers. The linear polymer amylose can be defined as the most basic form of starch, while amylopectin can be defined as the branched form.
Therefore, Starch is a covalent compound because of the covalent bonds between the glucose molcules.
Learn more about the covalent compound, here:
brainly.com/question/12144907
#SPJ4
plz help this is due today........

Answers
3 Stearic acid is a solid at room temperature.
The diagram below shows the apparatus used for finding the melting point of stearic acid.
The apparatus was heated at a steady rate and the temperature recorded every minute.
b Suggest why the water needs to be kept stirred during this experiment.
Answers
The water needs to be kept stirred during the experiment to ensure uniform and consistent heat transfer to the stearic acid.
Stirring the water helps distribute the heat evenly throughout the water bath. This prevents any localized temperature variations that could affect the accuracy of the recorded melting point of the stearic acid.
By keeping the water stirred, the heat is uniformly transferred to the stearic acid sample, promoting a more reliable and accurate determination of its melting point. Without stirring, there could be variations in temperature within the water bath.
Learn more about stirring, here:
https://brainly.com/question/29356443
#SPJ1
which of the following statements is false? (a) the properties of n2(g) will deviate more from ideality at -100oc than at 100oc. (b) van der waal's equation corrects for the non-ideality of real gases. (c) molecules of ch4(g) at high pressures and low temperatures have no attractive forces between each other. (d) molecules of an ideal gas are assumed to have no significant volume. (e) real gases do not always obey the ideal gas laws.
Answers
Molecules of ch4(g) at high pressures and low temperatures have no attractive forces between each other is a false statement.
Increasing the pressure and decreasing the temperature decreases the average distance between both the molecules, so the volume of molecules and their interaction must be considered.
Various theories explain the nature of gases and how other factors such as pressure, volume, and temperature influence the character of gases. The expressions relating volume, pressure, temperature, and number of moles are connected by the equations known as an ideal gas equation, Boyle's law, Charles' law, and Gay-lussac's law.
Only at Elevated temperature and relatively low pressure do real gases function like ideal gases. For ideal gases, we recognize intermolecular forces to be negligible and the size of individual gas molecules to be significantly smaller than intermolecular distances.
For more information on Temperature and pressure, visit :
https://brainly.com/question/1104898
#SPJ4