Answers
Answer:
D. create metal works of art. The bronze age was the age of crafting out of metal. So, using swords of metal would fit directly into the bronze age as they're metal.
Related Questions
what happens if you add nitric acid with hydrogen peroxide
Chem 101
Help
Answers
nitric oxide reacts with hydrogen peroxide to release large amounts of chemiluminescence with the characteristics of the highly cytotoxic species, singlet oxygen.
, singlet oxygen.Explanation:This is supported by the observation that when nitric oxide was added to a superoxide generating system, catalase inhibited the production of singlet oxygen while superoxide dismutase enhanced it
What is citric acid formula and uses?
Answers
The chemical formula for citric acid is C6H8O7. Citric acid is a weak organic acid found naturally in citrus fruits such as lemons and limes. It is also used as an ingredient in many foods, drinks, and dietary things.
Citric acid has many uses. It is used as a flavoring agent, preservative, and pH adjuster. It is also used in cleaning and laundry products, metal cleaning products, and cosmetics. In addition, it is used in pharmaceuticals and in treating kidney stones. Citric acid is also used as a chelating agent, to bind metal ions in solution, and as a sequestrant, to prevent minerals such as calcium and magnesium from forming insoluble precipitates.
To learn more about acid click here https://brainly.com/question/14072179
#SPJ4
what is the ph of a 1.66×10-2 m solution of the strong acid hbr? hbr(aq) h2o(ℓ) → br-(aq) h3o (aq) [hbr]0 = 1.66×10-2
Answers
The pH of a 1.66×\(10^{-2}\) M solution of the strong acid HBr is 1.78. The pH of a 1.66×\(10^{-2}\) M solution of the strong acid HBr can be determined using the equation pH = -log[\(H_3O^+\)].
Since HBr is a strong acid, it will completely dissociate in water to produce \(H_3O^+\) and Br- ions. Therefore, the concentration of \(H_3O^+\) ions will be equal to the initial concentration of HBr, or 1.66×\(10^{-2}\) M.
Using the equation pH = -log[\(H_3O^+\)], we can plug in the concentration of \(H_3O^+\) ions to find the pH:
pH = \(-log(1.66.10^{-2}) = 1.78\)
Therefore, the pH of a 1.66×\(10^{-2}\) M solution of the strong acid HBr is 1.78.
Here you can learn more about pH calculation https://brainly.com/question/30255888
#SPJ11
The molar heat of fusion of strontium metal is 7.43 kJ/mol, whereas its heat of vaporization is 137 kJ/mol.
Why is the heat of vaporization so much larger than the heat of fusion?
a.Since conversion from a liquid to a gas breaks many more intermolecular forces than conversion from a solid to a liquid, it requires much more energy.
b.Since conversion from a solid to a liquid breaks many more intermolecular forces than conversion from a liquid to a gas, it requires much less energy.
c.Since conversion from a solid to a liquid breaks many more intermolecular forces than conversion from a liquid to a gas, it requires much more energy.
d.Since conversion from a liquid to a gas breaks many more intermolecular forces than conversion from a solid to a liquid, it requires much less energy.
What quantity of heat would be needed to melt 1.00 g strontium at its normal melting point?
Heat = J
What quantity of heat would be needed to vaporize 1.00 g strontium at its normal boiling point?
Heat = J
What quantity of heat would be evolved if 1.00 g strontium vapor condensed at its normal boiling point?
Heat = J
Answers
c. Since conversion from a solid to a liquid breaks many more intermolecular forces than conversion from a liquid to a gas, it requires much more energy. 7.43 J, 137 J, 137 J.
The heat of vaporization is larger than the heat of fusion because the conversion from a liquid to a gas involves breaking many more intermolecular forces. In the liquid state, the particles are still relatively close together and have strong intermolecular forces holding them together. To convert the liquid into a gas, these intermolecular forces need to be overcome, requiring a significant amount of energy.
On the other hand, the conversion from a solid to a liquid involves breaking fewer intermolecular forces. In the solid state, the particles are already in close proximity, and the intermolecular forces are relatively weaker than those in the liquid state. Therefore, it requires less energy to melt a solid and convert it into a liquid.
Overall, the heat of vaporization is larger than the heat of fusion because the conversion from a liquid to a gas involves a more significant disruption of intermolecular forces, requiring more energy to overcome.
To calculate the quantity of heat needed to melt 1.00 g of strontium at its normal melting point, you would use the molar heat of fusion (7.43 kJ/mol) to convert grams to moles and then calculate the heat required using the molar heat of fusion value.
Similarly, to determine the quantity of heat needed to vaporize 1.00 g of strontium at its normal boiling point, you would use the molar heat of vaporization (137 kJ/mol) to convert grams to moles and calculate the heat required.
If 1.00 g of strontium vapor were to condense at its normal boiling point, the same quantity of heat that was absorbed during vaporization (137 kJ/mol) would be released.
To learn more about intermolecular forces click here: brainly.com/question/31797315
#SPJ11
The weather instruments: rain gauge can measure the amount of snow fall
True or false
Answers
For fatty acids with the same number of carbon atoms, how does the melting point change as the number of double bonds in the fatty acid changes?
Answers
For fatty acids with the same number of carbon atoms, the melting point change as the number of double bonds in the fatty acid changes there will be an increase in the melting point.
What is a melting point?The melting point of a substance it the temperature at which any substance of matter starts to melt then the measured temperature is said to be the melting point.
The melting point of the number of double bonds in the fatty acid is increased as needs more energy to break the bond of a carbon atom.
Therefore, there will be an increase in the melting point. if fatty acids with the same number of carbon atoms, the melting point change as the number of double bonds.
Learn more about melting point, here:
https://brainly.com/question/20551307
#SPJ4
name two substances that undergo melting
Answers
Answer:
they ate lelo pudina hahahha
Suppose that a beaker of water is 15°C and you raise the
temperature by 5°C. Use the graph above to calculate the percent decrease in the amount of dissolved O2 gas.

Answers
The percentage decrease in the amount of dissolved oxygen is 10%
Percent yield is the percent ratio of actual yield to the theoretical yield. It is calculated to be the experimental yield divided by theoretical yield multiplied by 100%. If the actual and theoretical yield are the same, the percent yield is 100%
In chemistry, yield is a measure of the quantity of moles of a product formed in relation to the reactant consumed, obtained in a chemical reaction, usually expressed as a percentage.
From the graph,
The amount of dissolved oxygen at 15°C is 10 mg/L
The amount of dissolved oxygen at 20°C is 9 mg/L
The decrease in the amount of dissolved oxygen is 1mg/L
The percentage decrease = (1/10) × 100 = 10%
Learn more about Percentage yield, here:
https://brainly.com/question/30794636
#SPJ1
Which phrase defines bond energy? (1 point)
energy output when product bonds form
energy required to break a chemical bond
energy stored in chemical bonds
energy input needed to break reactant bonds
Answers
The phrase that defines bond energy is the energy required to break a chemical bond.
WHAT IS BOND ENERGY?Bond energy is amount of energy needed to break the atoms involved in a chemical bond into free atoms.
The bond energy of each molecule varies with the type of bond in the molecule. Stronger bonds require a higher bond energy and vice versa.
Therefore, the phrase that defines bond energy is the energy required to break a chemical bond.
Learn more about bond energy at: https://brainly.com/question/26141360
Answer:
D. Energy input needed to break bonds of reactants.
Explanation:
Activation energy is the minimum amount of energy required to initiate a chemical reaction by breaking the bonds of the reactants. It is the energy needed to reach the transition state, where the reaction can proceed, and new chemical bonds can be formed to create the products. The activation energy is the energy barrier that must be overcome for a reaction to occur.
Once the reactants have enough energy to overcome the activation energy, the reaction proceeds spontaneously, and energy is released when new bonds form in the products.
PS. I took the quick check.
Question 4(Multiple Choice Worth 2 points)
(01.05 LC)
What property of matter takes up space?
O Mass
O Temperature
O Volume
O Weight
Answers
Answer:
Its not mass
Explanation:
X Mass out.
A meteorite contains 0.17 g of nickel-59, a radioisotope that decays to form cobalt-59. the meteorite also contains 5.27 g of cobalt-59. How many nickel-59 half-lives have passed since the meteorite formed?
Answers
A radioisotope called nickel-59, which decays into cobalt-59, weighs 0.17 g in meteorites. 5.27 g of cobalt-59 are also present in the meteorite, meaning that 5 nickel-59 half-lives have transpired since the meteorite originated.
What is nickel is used for?Batteries, such as nickel-cadmium rechargeable batteries and nickel-metal hydride batteries used in hybrid cars, include nickel. Coinage has long used nickel as a metal. 25% nickel and 75% copper make up the five-cent "nickel" coin used in the United States. The atomic number 28 and symbol Ni are assigned to the chemical element nickel. A transition metal with ductility and hardness is nickel.
Which country is rich in nickel?After experiencing an anticipated 4.2% reduction in 2020 due to the COVID-19 pandemic, global nickel mine production increased to 2,427.4 thousand tonnes (kt) in 2021, an increase of 6.8% over the previous year. The top five nations that produced nickel in 2021 were Australia, Indonesia, the Philippines, New Caledonia, and Russia.
Mass of nickel-59 = 0.17 g
Mass of cobalt-59 = 5.27 g
The nickel-59 radioactive decay equation is:
59/58 Ni→59/27 Co+_(-1)^0 β
The stoichiometry of reaction and the moles of reactant and product are now being used to compute the starting amount of nickel-59.
Formula used : Moles=(Given Mass)/(Molar Mass)
Moles of 59/28 Ni=0.17g/(59g/mol)=0.00288moles
Moles of 59/27 Ni=5.27g/(59g/mol)=0.089moles
By stoichiometry of the reaction,
1 mole of 59/28 Ni yields 1 mole of 59/27 Co.
Hence, the process will provide 0.089 moles of 59/27 Co.
=1/1×0.089=0.089moles of 59/28 Ni
Amount of 59/28 Ni decomposed will be = 0.089 moles
Initial amount of 59/28 Ni will be = Amount decomposed + Amount left = (0.00288 + 0.089)moles = 0.09188 moles
Now, we apply the following formula to get the number of half lives:
a=a_°/2^n
where,
a = the quantity of reactant remaining after n-half lifetimes.
= 0.00288 moles
= Initial amount of the reactant
= 0.09188 moles
n = number of half lives
After entering all the values into the equation above, we get
0.00288=0.09188/2^n
2^n=32.81
By dividing the log in half, we obtain
n log2 = log (32.81)
n = 5.03 = 5
Hence, since the meteorite created, '5' number of half-lives had transpired.
To know more about Nickel visit:
https://brainly.com/question/27362993
#SPJ4
Anyone know the answer to this? Explain if you do, im not sure if i understand what its asking.

Answers
Answer:
1. Proton = 4
2. Neutron = 5
3. Electron = 2
Explanation:
9 4Be^2+
From the above, we obtained the following:
Atomic number = 4
Mass number = 9
Charge = +2
1. Determination of the proton.
Atomic number is simply defined as the proton number. This implies that:
Atomic number = proton number
Atomic number = 4
Therefore,
4 = proton number
Proton = 4
2. Determination of the Neutron.
Mass number = Proton + Neutron
Mass number = 9
Proton = 4
Neutron =?
Mass number = Proton + Neutron
9 = 4 + Neutron
Collect like terms
Neutron = 9 – 4
Neutron = 5
3. Determination of the electron.
From the question given
9 4Be^2+
The atom has a charge of +2. This implies that the atom has lose 2 electrons. Thus we can obtain the electron as follow:
Proton = 4
Charge = +2
Electron =?
Electron = Proton – charge
Electron = 4 – 2
Electron = 2
How many moles of calcium chloride are needed to produce 0.78 moles of calcium nitrate?
Answers
0.0026 mol of calcium chloride are needed to produce 0.78 moles of calcium nitrate.
Molar mass is described as the mass in grams of 1 mole of a substance. The devices of molar mass are grams in line with mole, abbreviated as g/mol.
The mass of an isotope of any given element The quantity of moles of a substance equals the ratio of its given mass in a chemical response to the mass of 1 mole of that substance. One mole of any substance equals Avogadro's quantity, that is, 6.023 × 1023. It is likewise used to explicit attention devices which include mole in line with litre or molecular weight.
1 mol CaCl2 produces 2 mol CaCl2
Molar mass CaCl2 110.98 + 35.45 = 146.43 g/mol
Mol Ca(NO3)2in 0.78 g = 0.78 g / 146.43 g/mol = 0.0053 mol
This will require 0.0053/2 = 0.0026 mol
Answer to your question: 0.0026 mol
Learn more about Moles here https://brainly.com/question/16488605
#SPJ9
at what points does the object accelerate please explain
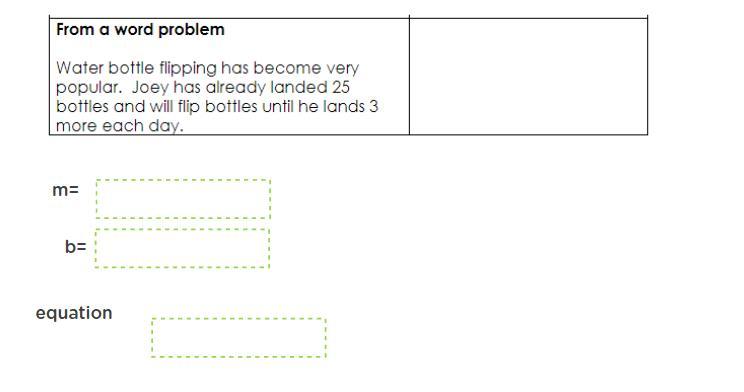
Answers
Answer:
M=3
B=25
Equation= 3x+25
Explanation:
M is your slope and if you follow the slope- intercept formula (y=m+b) you just need to plug in the numbers.
Thus your answer:
M=3
B=25
Equation= 3x+25
The rate constant for this first‑order reaction is 0.0830 s−1
at 400 ∘C.
A⟶products
After how many seconds will 16.8%
of the reactant remain?
Answers
The reaction has not yet started, and the time required for 16.8% of the reactant to remain cannot be calculated using the given rate constant.
Given,The rate constant for this first-order reaction is 0.0830 s−1 at 400 ∘C.A⟶products After how many seconds will 16.8% of the reactant remain-The time taken for a first-order reaction to reach a particular percentage of completion can be calculated using the following formula:t = (ln(A/A₀))/kwhere A₀ is the initial concentration of the reactant, A is the concentration of the reactant at a given time, k is the rate constant, and t is the time elapsed since the reaction began.In this question, we are given the rate constant, k = 0.0830 s−1 at 400 ∘C, and we want to find out the time required for 16.8% of the reactant to remain.Let's assume that the initial concentration of the reactant is 100 units (we can assume any value as it does not affect the percentage of completion).Therefore, the concentration of the reactant remaining after 16.8% of completion would be: A = 16.8 units.Substituting these values in the above formula, we get:t = (ln(16.8/100))/0.0830t = (−1.7918)/0.0830t = −21.58 sThis time value is negative, which means that the reaction has not even started yet. Therefore, we need to check the given percentage of completion.
If it is less than 50%, we can assume that the reaction has not yet started. In this case, the percentage of completion is 16.8%, which is less than 50%.
for such more questions on reaction
https://brainly.com/question/24795637
#SPJ8
what is the energy of a purple lamp with a frequency of 7.5×10^14hz
Answers
Answer:
4.97x10^-19 Joules
Explanation:
Energy and frequency are related by Planck's constant.
Energy = (Planck's Constant)*(Frequency)
Planck's constant = 6.626x10^-14 J/Hz
Energy = (6.626x10^-34 J/Hz)*(7.5x10^14 Hz)
Energy = 4.97x10^-19 J
Which of the substances are elements please help

Answers
Answer:
Substances 1 and 2
Explanation:
an element only has 1 kind of atoms :3
Expplain why rooms upstairs or in the attic are often the warmmest plpaces in the house.Make sure to refare to conduction convection radiation in your explanation
Answers
The rooms upstairs or in the attic are often the warmest places in the house because of heat transfer by convection (rising of hot air), conduction (heating of the attic level by the ceiling) and radiation (transfer of heat rays from attic floor to the roof).
What is heat transfer by convection?Heat transfer by convection is the process of heat transfer by the bulk movement of molecules within fluids such as gases and liquids.
Convection current occurs when warm or hot rises up and cool air with higher density moves down to replace the hot risen air.
The rooms upstairs or in the attic are often the warmest places in the house because warm air in the home rises due to heat transfer by convection.
The heat from the hot ceiling is transferred to the attic level by conduction heat transfer process. The roof also receives heat radiation from the attic floor.
Thus, the rooms upstairs or in the attic are often the warmest places in the house because of heat transfer by convection (rising of hot air), conduction (heating of the attic level by the ceiling) and radiation (transfer of heat rays from attic floor to the roof).
Learn more about heat transfer process here: https://brainly.com/question/16055406
#SPJ1
What is the difference between metoprolol tartrate and metoprolol succinate.
Answers
Answer:
Metoprolol tartrate is the immediate-release version of metoprolol while metoprolol succinate is the extended-release version
Explanation:
This means that metoprolol succinate is released over time in the body leading to longer-acting effects. Metoprolol tartrate may need to be taken multiple times per day.
What make up molecules
Answers
Answer:
atom
Explanation:
because the definition of molecule is the group of an atom is called molecule
Answer:
Molecules are made up of One Or More atoms.If they contain More than One atom,the atom can be the Same Or different.
Is freezing point depression a colligative property?
Answers
Answer:
Yes it is a colligative Property
Explanation:
There is freezing point depression, boiling point elevation, Osmotic Pressure, Vapor Pressure Lowering
which of the following is not a property of carbon?group of answer choicesit can form single, double, and even triple bonds with itself.all compounds made from carbon are soluble in water.it can be built into rings and long chains.all organic molecules contain carbon atoms.
Answers
All compounds made from carbon are water soluble, which is not a property of carbon.
Due to its highly stable structure and the bonds formed, carbon is insoluble in any solvent. Carbon needs other atoms attached to it to become polar enough to dissolve in water. For example, carbon does not dissolve in water, but carbon dioxide (CO2) does. Most organic compounds are nonpolar and therefore do not mix with polar molecules such as water. Therefore, organic matter is generally insoluble in water. All compounds made from carbon are water soluble, which is not a property of carbon. Elemental carbon is an inert substance, insoluble in water, dilute acids and bases, and organic solvents. At high temperatures, it combines with oxygen to form carbon monoxide or carbon dioxide.
Learn more about carbon
brainly.com/question/19886129
#SPJ4
Rowena and helga are both performing an experiment with nickel metal. Rowena has a 5 gram sample and determines the density to be 8.9g/cm3. If helga has a nickel sample that is twice as large and has a mass of 10 grams what would be the density of helgas sample?
Answers
Answer:
The density of helgas sample is 17.8 g/cm³.
Explanation:
Given that,
Mass of sample of Rowena = 5 gram
Density = 8.9 g/cm³
Mass of sample of helga = 10 gram
We need to calculate the volume of sample
Using formula of volume
\(V=\dfrac{m}{\rho}\)
Where, m = mass
\(\rho\) = density
Put the value into the formula
\(V=\dfrac{5}{8.9}\)
\(V=0.56\ cm^3\)
We need to calculate the density of helgas sample
Using formula of density
\(\rho=\dfrac{m}{V}\)
Where, m = mass
V = volume
Put the value into the formula
\(\rho=\dfrac{10}{0.56}\)
\(\rho=17.8\ g/cm^3\)
Hence, The density of helgas sample is 17.8 g/cm³.
1. Calculate the quantitative concentration of sodium chloride (NaCI) solution, which contains 0.3 mol of NaCI in 0.5 dm3
2. Determine the concentration of magnesium chloride solution (MgC12), which in 200 cm3
contains MgCl2.
3. How many grams of sodium hydroxide (NaOH)
needed to prepare 100cm3 of solution
mass concentration 80q/dm3?
Answers
2. 5.45 cm3
3. 70g NaOH
I turn the Ritz into a poor house
It's like eviction number four now
Go 'head and ash it on the floor now
Girl go 'head and show me how you go down
And I feel my whole body peaking
And I'm F anybody with they legs wide
NAME THE SONG
Answers
What forces typically hold ions together?
O A. Intermolecular forces
OB. Ionic attractions
OC. Metallic bonds
O D. Covalent bonds
Answers
Answer: Ionic attractions
Explanation:
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions.
4. A galvanic cell is formed when two metals are immersed in solu- tions differing in concentration 1 when two different metals are immersed ? when two different metals are exposed 1 j when two metals are brought close in one electrolyte to air together and electrically insulated from one another.
Answers
A galvanic cell is formed when two metals are immersed in solutions differing in concentration, when two different metals are immersed.
What is a Galvanic cell ?In order to provide a pathway for the flow of electrons along that wire, the galvanic cell makes use of the ability to split the flow of electrons during the oxidation and reduction processes. It forces a half-reaction and connects each to the other with a wire.A galvanic cell is an electrochemical device that converts chemical redox reaction energy into electrical energy. Electrically, it has a potential of 1.1 V. Oxidation takes place at the anode, which is a negative plate in galvanic cells. It is a positive plate where the reduction happens.An electrochemical device called a galvanic cell transforms chemical energy's free energy into electrical energy. A photogalvanic cell produces species that are photochemically reactive.To view more questions about galvanic cell, refer to:
https://brainly.com/question/13031093
#SPJ4
Which of the following is NOT a benefit of renewable energy? *
O generates power on smaller scale
O can decrease pollution
O combats climate change
Answers
Answer:
O generates power on a smaller scale
Explanation:
The other two options are benefits of renewable energy. You're looking for a con instead of a pro.
How does molecular shake affect polarity?

Answers
Answer:
I believe its A
Explanation:
A molecule that has a symmetrical shape will be a nonpolar molecule.
The roller coaster is your favorite ride! But the line is always long! Having just studied potential and kinetic energy in physics, you evaluate the energy changes of the cars as they move on the tracks. Compare the potential energy of the cars if they are stopped at points A to point D.
Answers
The comparison of the potential energy of the cars if they are stopped at points A to point D is C. Potential energy is stored energy, point D is taller so it has more stored energy.
What is a potential energy?Potential energy is the energy held by an object as a result of its position relative to other objects, internal stresses, electric charge, or other factors. Because any object lifted from its resting position has stored energy, it is referred to as potential energy because it has the potential to do work when released.
Kinetic energy is the energy that an object has as a result of its movement. If we want to accelerate an object, we must apply force to it. Using force requires us to put in effort. After work is completed, energy is transferred to the object, and the object moves at a new constant speed.
Learn more about kinetic energy on:
https://brainly.com/question/14427111
#SPJ1
The roller coaster is your favorite ride! but the line is always long! having just studied potential and kinetic energy in physics, you evaluate the energy changes of the cars as they move on the tracks. Compare the potential energy of the cars if they are stopped at points A to point D.
A. Neither have potential energy.
B. The potential energy is the same.
C. Potential energy is greatest at point D.
D. Potential energy is greatest at point A.

