With a dialysis bag that is impermeable to sucrose, a mass change of a beaker (with 0.1M sucrose) containing a dialysis tubing with 0.8M sucrose is due to
Answers
The mass change of the beaker containing a dialysis tubing with 0.8M sucrose, enclosed by a dialysis bag impermeable to sucrose, is due to the movement of water molecules through the dialysis bag.
The sucrose concentration inside the dialysis tubing is higher (0.8M) compared to the sucrose concentration in the beaker (0.1M). This creates an osmotic gradient, where water molecules tend to move from an area of lower solute concentration (the beaker) to an area of higher solute concentration (inside the dialysis tubing).
As water molecules move across the dialysis bag, the beaker experiences a net loss of water, resulting in a decrease in mass. The sucrose molecules, being too large to pass through the impermeable dialysis bag, remain inside the tubing.
This process, known as osmosis, continues until the sucrose concentrations inside and outside the dialysis tubing reach equilibrium. At this point, the mass change of the beaker will stabilize, and no further net movement of water will occur.
To know more about osmosis , refer here:
https://brainly.com/question/31028904#
#SPJ11
Related Questions
The picture above is a diagram of what earth-sun-moon phenomena?
A.Solar System
B.Solar Eclipse
C.Lunar Eclipse
D.Earth-Sun-Moon
If you don't know plz don't answer

Answers
que es y que define a los elementos quimicos
Answers
Un elemento químico, o un elemento, se define como un material que no puede descomponerse ni transformarse en otra sustancia por medios químicos. Cada elemento se identifica según la cantidad de protones que tiene en su núcleo atómico. Se puede crear un nuevo elemento agregando más protones a un átomo.
_________
Espero eso ayude ⚜
ITZY :)
in this experiment, increasing the concentration of lactose beyond 500 mg/dl does not increase the rate of reaction further. what is the best explanation for this phenomenon?
Answers
In this experiment, increasing the concentration of lactose beyond 500mg/dl does not increase the rate of reaction further because there are no free enzymes available to react with the lactose.
Initially, when the substrate concentration is increased, the rate of reaction also increases considerably and leads to an increase in the rate of an enzyme catalysed reaction. Now as the concentration of the substrate increases, there are no free enzymes available to react with these substrate. All the active sites of all the enzymes available are already occupied by the substrate molecules and further increasing the substrate molecules beyond this point lead to no additional effect as there are no free enzymes molecules available to carry out the reaction. Hence, increasing the concentration of lactose molecule does not increase the rate of reaction further. But if we add more lactose enzymes then the rate of the reaction will increase by decreasing the activation energy.
To learn more about rate of reaction, visit---
https://brainly.com/question/24795637
#SPJ4
A 0.80 L sample of gas has a temperature of 27°C and a pressure of 0.925 atm. How many moles of gas are present?
Answers
Answer:
n= 0.03 moles
Explanation:
Using the ideal gas law:
PV=nRT
nRT=PV
n= PV/RT
n: moles
P: pressure in atm
V= volume in L
R= Avogadro's constant = 0.0821
T= Temperature in K => ºC+273.15
n= (0.925 atm)(0.80 L) / (0.0821)(300.15 K)
n= 0.03 moles
What energy-storing molecule is broken down in the first step of cellular respiration?
Answers
Glucose is broken down in the first step of cellular respiration.
Glycolysis, the first stage of cellular respiration, takes place in the cell cytoplasm. It involves the splitting of glucose into two three-carbon molecules through a 10-step process divided into two phases. The first phase involves the division of a six-carbon sugar molecule, while the second phase extracts and stores energy in the form of ATP and NADH. These two phases are known as the energy investment and energy generation phases. The overall net energy gain from this process is two ATP.
If you need to learn more about cellular respiration, click here
https://brainly.com/question/13721588?referrer=searchResults
#SPJ4
why does the atmosphere weigh so much
Answers
Answer:
Because the Earth's gravity acts on the atmosphere and holds it down, just as it does all other objects. The weight of the atmosphere is estimated to be about 5.75 quadrillion (5,750,000,000,000,000) tons. Air pressure is the "push" of the atmosphere on the Earth's surface.
What is the law of definite proprtions and how does it apply to this experiment
Answers
According to law of definite proportions, chemical compound always includes its constituent components in a fixed ratio.
What is law of definite proportions?The law of definite proportions, also known as Proust's law or the law of constant composition, holds that no matter the source or technique of manufacture, a particular chemical compound always includes its constituent components in a fixed ratio.
When two elements are combined to form a compound, their mass proportions are always the same. For instance, oxygen makes up roughly 8/9 of both the mass of just about any samples of pure water, and hydrogen make up the remaining 1/9.
Therefore, according to law of definite proportions, chemical compound always includes its constituent components in a fixed ratio.
To know more about law of definite proportions, here:
https://brainly.com/question/30051084
#SPJ1
What is the stoichiometric ratio of the following:
4FeS+7O2→2Fe2O3+4SO2
Answers
The stoichiometric ratio of \(4FeS + 7O_{2} → 2Fe_{2} O_{3} + 4SO_{2}\) is 4:7:2:4.
The stoichiometric ratio refers to the ratio of the amounts of reactants and products in a chemical reaction. In the given equation\(4FeS + 7O_{2} → 2Fe_{2} O_{3} + 4SO_{2}\), the stoichiometric ratio is 4:7:2:4, which means that for every 4 moles of FeS, 7 moles of \(O_{2}\) are required to produce 2 moles of \(Fe_{2} O_{3}\) and 4 moles of \(SO_{2}\).
This ratio is based on the balanced chemical equation, which indicates the number of molecules or moles of each reactant required to produce a certain amount of product. In this case, the equation shows that 4 moles of FeS react with 7 moles of \(O_{2}\) to produce 2 moles of \(Fe_{2} O_{3}\) and 4 moles of \(SO_{2}\).
Understanding stoichiometry is important in chemistry as it allows chemists to predict the amount of product that can be obtained from a given amount of reactant and vice versa. It also helps in determining the limiting reactant in a reaction, which is the reactant that limits the amount of product that can be formed.
Learn more about molecules here:
https://brainly.com/question/30579952
#SPJ4
Is NaCl a nonpolar covalent bond?.
Answers
No, NaCl is not a nonpolar covalent bond. NaCl, also known as table salt, is an ionic compound, not a covalent compound.
Ionic compounds are formed when a metal and a nonmetal combine, and the bond between them is an ionic bond. In the case of NaCl, the sodium atom donates an electron to the chlorine atom, creating a positive ion (Na+) and a negative ion (Cl-), which are held together by the electrostatic attraction between the ions.
Covalent compounds, on the other hand, are formed when nonmetal atoms share electrons in order to fill their valence shells. Covalent bonds can be polar or nonpolar. A polar covalent bond occurs when the electrons are not shared equally between the atoms. In contrast, a nonpolar covalent bond occurs when the electrons are shared equally between the atoms, creating a symmetrical distribution of electron density.
In summary, NaCl is an ionic compound formed by the combination of a metal (Na) and a nonmetal (Cl) by an ionic bond, not a covalent bond, and it is not a polar or nonpolar covalent bond.
Learn more about covalent bond:
https://brainly.com/question/9833711
#SPJ4
is gasoline a heterogeneous mixture o
Answers
Distinguishing Between Pure Substances and Mixtures
Pure substances can be either elements or compounds. Mixtures can be either homogeneous or heterogeneous. ... Gasoline is thus a blend of different compounds, so it is a mixture.
A student makes several observations about a plece of iron. Which observation describes a chemical property of the Iron?
Answers
Answer:rust
Explanation:
Construct the requested confidence interval. A survey of 400 non-fatal accidents revealed that 268 involved a distracted driver using some kind of electronic device. Construct a 95% confidence interval for the proportion of non-fatal accidents involving a distracted driver using some kind of electronic device.
Answers
Approximately (0.628, 0.712) is the 95% confidence range for the percentage of non-fatal accidents involving a distracted driver utilising some type of electronic gadget.
The following formula can be used to create a confidence interval for the percentage of non-fatal incidents involving a distracted driver using an electronic device:
\(\[ \text{{Confidence Interval}} = \hat{p} \pm z \cdot \sqrt{\frac{\hat{p}(1-\hat{p})}{n}} \]\)
where:
- \(\(\hat{p}\)\) is the sample proportion in this case (268/400).
- \(\(n\)\) the sample size, which in this case is 400,
- \(\(z\)\) is the z-score that the chosen confidence level corresponds to.
For a 95% confidence interval, the corresponding z-score is approximately 1.96.
When the values are added to the formula, we obtain:
\(\[ \text{{Confidence Interval}} = \frac{268}{400} \pm 1.96 \cdot \sqrt{\frac{\left(\frac{268}{400}\right) \left(1 - \frac{268}{400}\right)}{400}} \]\)
Simplifying the equation:
\(\[ \text{{Confidence Interval}} = 0.67 \pm 1.96 \cdot \sqrt{\frac{0.67 \cdot 0.33}{400}} \]\)
Calculating the values:
\(\[ \text{{Confidence Interval}} = 0.67 \pm 1.96 \cdot \sqrt{\frac{0.2211}{400}} \]\[ \text{{Confidence Interval}} = 0.67 \pm 1.96 \cdot 0.0210 \]\[ \text{{Confidence Interval}} = 0.67 \pm 0.0412 \]\)
Therefore, Approximately (0.628, 0.712) is the 95% confidence range for the percentage of non-fatal accidents involving a distracted driver utilising some type of electronic gadget.
To learn more about electronic configuration from the given link
https://brainly.com/question/26084288
#SPJ4
What is the overall charge of the electron cloud of the atom? Explain.
Answers
Answer:
Explanation:
What does this mean? It means that there are Z positive nuclear charges, and this determines the identity of the element. If the element is neutral, this means that there are precisely Z electrons, and the overall electronic charge is −Z . And thus the net ELECTRIC charge of the ATOM is Z+(−Z)=0 , i.e. zero .Oct 3, 2016
the density of mercury is 13.6 g/cm3 what is the mass of 8.20 ml of mercury
Answers
Answer:
The answer is
111.52 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
density of mercury = 13.6 g/cm³
First we must convert the volume from mL to cm³
if 1 mL = 1 cm³
Then 8.20 mL = 8.20 cm³
volume = 8.20 cm³
The mass of mercury is
mass = 13.6 × 8.2
We have the final answer as
111.52 gHope this helps you
What mass of methane gas (CH4) is required in order to follow the Law of
Conservation of Mass?

Answers
Answer:
2.3g
Explanation: my mom is a teacher
CH₄ + 2O₂ → CO₂ + 2H₂O For this reaction the mass of methane gas (CH4) is required in order to follow the Law of Conservation of Mass is 2.3 gram. Therefore, option D is correct.
What is the Law of Conservation of Mass ?The Law of Conservation of Mass states that the total mass of the reactant is exactly equal to the total mass of the product.
It is also state that in a chemical reaction mass is neither created nor destroyed. For example, the carbon atom in coal gets carbon dioxide when it is burned.
This law is important in understanding and production of various chemical reactions. If researchers experience the identities alongside the quantities of the reactants for any particular reaction.
According to the Law of Conservation of Mass, In given combustion reaction to obey this law the mass of reactant is 2.3 grams.
Thus, option D is correct.
To learn more about the Law of Conservation of Mass, follow the link;
https://brainly.com/question/28711001
#SPJ6
How many moles are on a 7.0 cm x 10.0 cm sheet of 1.0 mm thick aluminum foil? The density of the material is 2.702 g/mL.
Answers
The number of mole present in the aluminum foil, given that the foil has a thickness of 1.0 mm is 0.7 mole
How do I determine the number of mole?We'll begin by obtaining the mass of the aluminum foil. Details below:
Density of aluminum = 2.702 g/mLDimension = 7 cm × 10 cm × 1 mm = 7 cm × 10 cm × 0.1 cmVolume of aluminum = 7 cm × 10 cm × 0.1 cm = 7 cm³ = 7 mLMass of aluminum =?Density = mass / volume
Cross multiply
Mass = Density × Volume
Mass of aluminum = 2.702 × 7
Mass of aluminum = 18.914 g
Finally, we shall determine the number of mole present. Details below:
Mass of aluminum = 18.914 gMolar mass of aluminum = 27 g/mol Number of mole of aluminum =?Mole = mass / molar mass
Number of mole of aluminum = 18.914 / 27
Number of mole of aluminum = 0.7 mole
Thus, the number of mole is 0.7 mole
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
what number of moles of h2 will be produced when 4.0 mol na is added to 1.2 mol h2o?
Answers
The balanced chemical equation for the reaction between sodium (Na) and water (H2O) is:
2Na + 2H2O → 2NaOH + H2
This means that for every 2 moles of sodium added, 1 mole of hydrogen gas (H2) is produced. Therefore, to calculate the number of moles of H2 produced, we need to first determine the number of moles of sodium added and then use the mole ratio from the balanced equation.
In this case, we are given that 4.0 moles of Na is added and 1.2 moles of H2O is present. Since Na and H2O react in a 1:2 ratio, we can determine the number of moles of NaOH produced by dividing the number of moles of H2O by 2:
1.2 mol H2O ÷ 2 = 0.6 mol NaOH
Since 2 moles of Na produce 1 mole of H2, we can use a mole ratio to calculate the number of moles of H2 produced:
4.0 mol Na × (1 mol H2 / 2 mol Na) =2.0 mol H2
Therefore, 2.0 moles of H2 will be produced when 4.0 mol Na is added to 1.2 mol H2O.
When 4.0 mol of Na reacts with 1.2 mol of H2O, the balanced chemical equation is:
2 Na + 2 H2O → 2 NaOH + H2
From the balanced equation, you can see that 2 moles of Na reacts with 2 moles of H2O to produce 1 mole of H2. To find the number of moles of H2 produced, first determine the limiting reactant:
Na: 4.0 mol / 2 = 2.0 (sets of reactants)
H2O: 1.2 mol / 2 = 0.6 (sets of reactants)
H2O is the limiting reactant. Now calculate the moles of H2 produced:
0.6 (sets of reactants) × 1 mol H2 = 0.6 mol H2
So, 0.6 moles of H2 will be produced when 4.0 mol of Na is added to 1.2 mol of H2O.
To know more about balanced chemical equation visit:
https://brainly.com/question/28294176
#SPJ11
sound waves travel fastest through
steel
air
water
Answers
Compared to air or water, steel has the fastest sound propagation speed.
What are sound waves?Vibrations known as sound waves travel through a medium like air, water, or solid things. As an object vibrates, the surrounding medium's molecules follow suit, causing a disturbance that travels across the medium as a wave of pressure changes. The human ear interprets these pressure changes as sound.
Solids, especially those with densely packed molecules and high densities, allow sound waves to travel the fastest. In contrast to air or water, sound waves move through steel the fastest.
In comparison to the speeds of sound in air and water, which are respectively 343 and 1,484 meters per second, respectively, in steel, the speed of sound is approximately 5,960 meters per second.
To know more about sound waves, visit:
https://brainly.com/question/11797560
#SPJ1
All systems disperse their energy spontaneously?
Is it true or false? and WHY?
Answers
Answer:
True.
Explanation:
Yes, all systems disperse their energy spontaneously because all types of energy spontaneously flows from concentrated to dispersed if it is not hindered by any material in their way. Energy can be prevented from spreading by materials such as containers. If there is no substance in the path of energy so it can disperse automatically from a concentrated region to a lower region.
Draw the structure that corresponds to the following molecular formula and proton decoupled 13C NMR-DEPT spectral data set (attached protons in parentheses):
Molecular Formula: C4H6O Spectral data: ? 27.2 (3H), ? 127.8 (2H), ? 136.4 (1H), ? 197.7 (zero H).
Answers
The structure of the compound with the molecular formula C4H6O and given spectral data is:
CH3 - CH = CH - C = O
Here's a step-by-step breakdown of the process:
1. From the molecular formula C4H6O, we can deduce that it's an unsaturated compound (Degree of Unsaturation = 2) since it has fewer hydrogens than the corresponding saturated compound C4H10. The two degrees of unsaturation indicate the presence of either two double bonds, one double bond and one ring, or one triple bond.
2. Analyzing the spectral data:
- δ 27.2 (3H): This peak represents a methyl (CH3) group attached to a sp3-hybridized carbon.
- δ 127.8 (2H): This peak indicates a carbon with a double bond (sp2-hybridized carbon) attached to two hydrogens (CH2=).
- δ 136.4 (1H): This peak represents a sp2-hybridized carbon with a double bond and one hydrogen (CH=).
- δ 197.7 (zero H): This peak indicates a carbonyl carbon (C=O).
3. With the information from the spectral data, we can construct the compound structure: CH3 - CH = CH - C = O
Summary: The structure corresponding to the molecular formula C4H6O and the given spectral data is CH3 - CH = CH - C = O, which is an unsaturated compound with one double bond and one carbonyl group.
Learn more about hydrogens click here:
https://brainly.com/question/24433860
#SPJ11
How do atoms bond in metallic bonding?
Answers
Answer:
the I really don't know sorry:(
What is the temperature and pressure at STP?
Answers
Answer:
Since 1982, STP is defined as a temperature of 273.15 K (0 °C, 32 °F) and an absolute pressure of exactly 105 Pa (100 kPa, 1 bar).
Explanation:
I hope I helped
The human _____ is like a ____
Answers
Answer:
The human BRAIN is like a MUSCLE
Explanation:
This common analogy is used to express the fact that the brain, like a muscle, can be trained and become better at what it does. Also, I don't think this is chemistry.
Answer:
brain muscle
Explanation:
9) Is there any other ratio of aluminum and oxygen ions that could exist?
For instance, could you have Alz0 or AlO2? Explain your answer.
Answers
Answer:
The ratio of aluminium and oxygen ions that only exists is 2:3
Since Aluminium has 3 valence electrons and oxygen has 2 vacant orbitals
Aluminium holds a valence of 3 and oxygen 2, when they react a compound of formula
\(Al _{2} O _{3}\)
is formed
According to the electronic configuration and valency, ratio that could exist between aluminium and oxygen is 2:3.
What is electronic configuration?Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/13497372
#SPJ2
why does adding hydrochloric acid to an aqueous solution of a carboxylic acid decreased its solubility in water?
Answers
Adding hydrochloric acid to an aqueous solution of a carboxylic acid decreases its solubility in water because of the common ion effect.
The common ion effect is the phenomenon in which the solubility of a salt is decreased when it is added to a solution containing an ion in common with the salt. In this case, the carboxylic acid and hydrochloric acid both contain the H+ ion. When hydrochloric acid is added to the solution, the concentration of H⁺ ions increases, causing the carboxylic acid to become less soluble in water.
This can be explained by Le Chatelier's principle, which states that when a system at equilibrium is subjected to a stress, the system will shift in a direction that reduces the stress. In this case, the addition of hydrochloric acid increases the concentration of H⁺ ions, causing the system to shift in a direction that reduces the concentration of H+ ions. This is achieved by decreasing the solubility of the carboxylic acid in water, causing it to precipitate out of the solution.
know more about hydrochloric acid here
https://brainly.com/question/15231576#
#SPJ11
how does one determine a molecular formula from the epirical formula
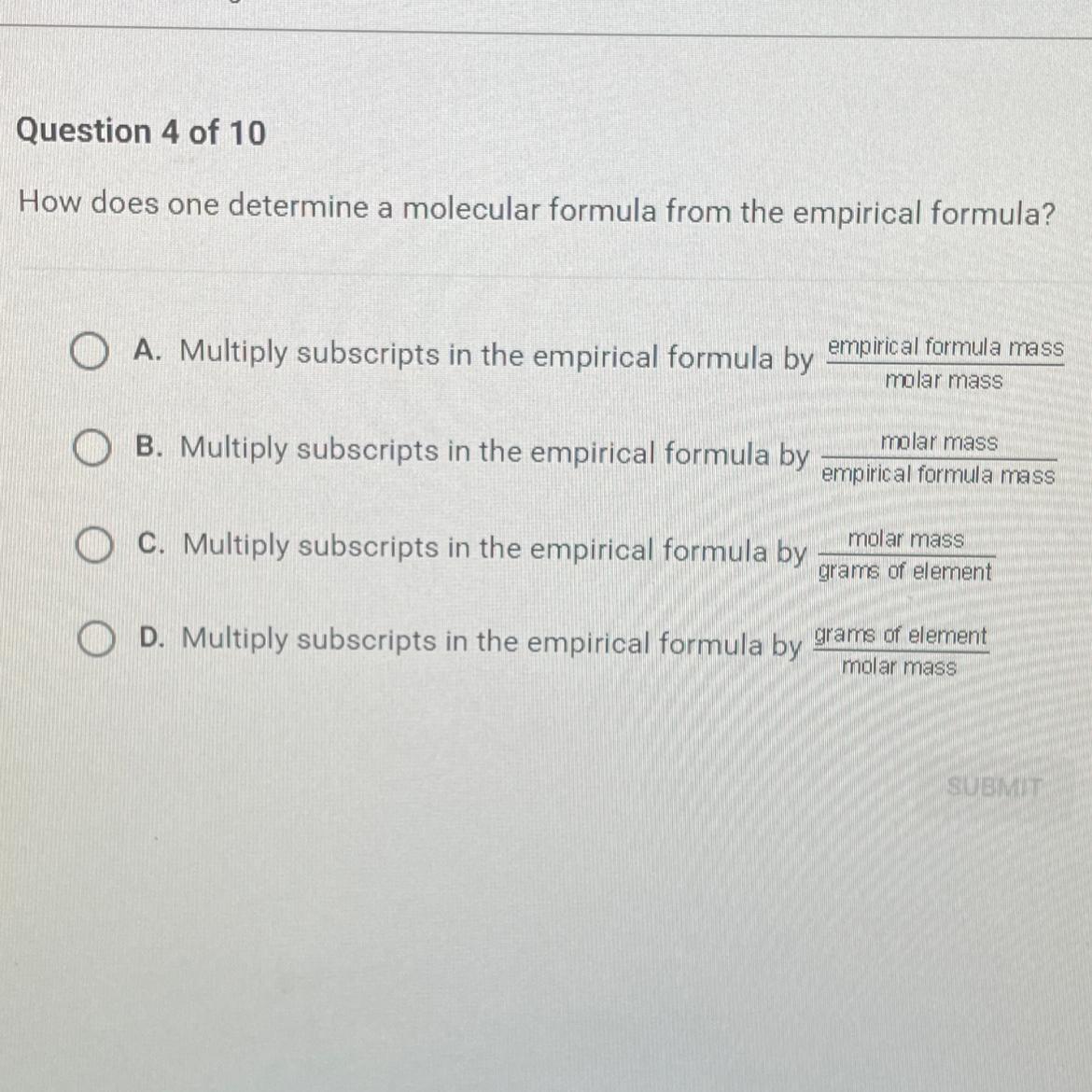
Answers
Answer:
C
Explanation:
Help me please. Which type of plate boundary is responsible for volcanoes and mountain building?
A. convergent
B. divergent
C. transform
Answers
Answer:
i should be A i hope this helps
Explanation:
Calculate the amount of calcium carbonate (mg) not in solution if a tablet is dissolved in 250 ml of water and the tablet’s label claim is 600 mg calcium / tablet.
Answers
Therefore, the amount of calcium carbonate that is not in solution in the tablet is approximately 596.75 mg.
If the tablet's label claim is 600 mg calcium per tablet and the tablet is dissolved in 250 ml of water, we need to calculate the amount of calcium carbonate that is not in solution.
To do this, we first need to know the molecular weight of calcium carbonate, which is 100.09 g/mol. We can then convert the amount of calcium claimed on the label to milligrams per milliliter (mg/mL) by dividing by the volume of water used:
600 mg / 250 mL = 2.4 mg/mL
Next, we need to determine the solubility of calcium carbonate in water. Calcium carbonate is sparingly soluble in water, meaning that only a small fraction of it will dissolve. According to the CRC Handbook of Chemistry and Physics, the solubility of calcium carbonate in water at 25°C is 0.0013 g/100 mL. This corresponds to a concentration of 0.013 mg/mL.
Therefore, the amount of calcium carbonate that is not in solution can be calculated by subtracting the solubility from the total amount of calcium in the tablet:
2.4 mg/mL - 0.013 mg/mL = 2.387 mg/mL
Multiplying this by the total volume of water used:
2.387 mg/mL x 250 mL = 596.75 mg
Therefore, the amount of calcium carbonate that is not in solution in the tablet is approximately 596.75 mg.
To know more about calcium carbonate visit:
https://brainly.com/question/13565765
#SPJ11
An man in Arkansas recently found a 9 carat diamond at Crater of Diamonds State Park. Five carats are equivalent to one gram, so this diamond weighs 1.8 g. Diamond is a crystalline form of the element carbon. How many atoms of carbon are in this 1.8 g diamond
Answers
Answer:
The answer is "1.8 g diamond includes 9.03e22 atoms".
Explanation:
Given:
Weight of 9-carat diamond = 1.8 g
\(\therefore\\\\\)
weight of 1-mole carbon =12 g
\(\because\)
calculating the moles which are available into 18 g carbon weight:
x= Carbon moles weight in 1.8 g
\(1 - mol\ C = 12 g\\\\x - mol\ C = 1.8 g\\\\\to x = \frac{(1.8 \ g \times 1\ mol\ C)}{ 12 \ g}\\\\\to x = 0.15\ moles\)
In 1 mole element associated with Avogadro's number that is \(6.02 \times 10^{23}\\\\\)
Carbon includes in 1 mol\(=6.02 \times 10^{23}\ atoms\\\\\)
0.15 mol of carbon includes:
\(= 0.15 \times 6.02 \times 10^{23} = 9.03 \times 10^{22}\ atoms.\)
Using the scientific notation:
0.15 mol of carbon includes: 9.03e22 atoms. So, 1.8 g diamond includes 9.03e22 atoms.
Determine the kinds of intermolecular forces that are present in each element or compound.
1. Kr a. dispersion forces b. dipole-dopole forces
c. hydrogen bonding d. dispersion forces & dipole-dipole forces
2. NH3 a. dispersion forces b. dipole-dopole forces
c. hydrogen bonding d. dispersion forces & dipole-dipole forces
3. NO a. dispersion forces b. dipole-dopole forces
c. hydrogen bonding d. dispersion forces & dipole-dipole forces
4. CH4 a. dispersion forces b. dipole-dopole forces
c. hydrogen bonding d. dispersion forces & dipole-dipole forces
Answers
The kinds of intermolecular forces that are present in each element or compound are as follows:
1. Kr - dispersion forces
2. NH3 - dispersion forces, dipole-dipole forces and hydrogen bonding
3. NO - dispersion forces and dipole-dipole forces
4. CH4 - dispersion forces
Let's understand this in detail:
The electronegativity of the atom in question and the relative proximity of the atom to other atoms determines the strength of the intermolecular forces.
In Kr, the intermolecular forces present are the weakest type, dispersion forces. This is because Kr is a noble gas with the lowest electronegativity among the elements.
In NH3, stronger dispersion forces, dipole-dipole forces, and hydrogen bonding are present because nitrogen has a high electronegativity and is surrounded by three hydrogen atoms with partial positive charges due to their electron-deficient nature.
In NO, there are only dispersion and dipole-dipole forces since no hydrogen atom is present to form hydrogen bonding.
Finally, in CH4, only dispersion forces are present since the four hydrogen atoms do not form a tetrahedral structure due to their symmetrical electron distribution.
#SPJ11
Learn more about intermolecular forces: Define Intermolecular forces https://brainly.com/question/12243368