Answers
Answer:
a. C > F > Cl
Explanation:
We know that atomic mass of Chlorine is greater than of Florine than that of carbon. Moreover, in CF2Cl2, therefore, there are two atoms of Cl, F and one atom of C. Therefore, in CF2Cl2 in order of decreasing mass percent composition C > F > Cl. Therefore, the correct option is a.
Related Questions
Fill in the blank to complete each statement
The land that supplies water to a river system is called a
Pollution from a single, identifiable source is called
Pollution that is difficult to link to a particular origin is called
source pollution
source pollution.
Answers
Answer:
1- Watershed
2- Point
3- Nonpoint
Explanation:
Just did it
Answer:
1. watershed
2. point
3. nonpoint
Explanation:

Calculate the solubility product for the following:
Ce(IO3)4, 1.5 × 10–2 g/100.0 mL (IO3- is the anion)
Ksp = ______ x10______
Use correct significant figures
Answers
Answer: 2.749 x 10^-14
Explanation:
Given:
The concentration of Ce(IO3)4 in the solution = 1.5 x 10-2 g/100 mL= 0.15 g/1000 mL= 0.15 g/L
Molar mass of Ce(IO3)4 = 839.7267 g/mol
Therefore, the molarity of Ce(IO3)4 in the solution is given by:
\($$\begin{gathered}M=\frac{0.15 \mathrm{~g} / L}{839.7267 \mathrm{~g} / \mathrm{mol}} \\M=1.7863 \times 10^{-\mathbf{4}} \mathrm{mol} / {L}=\mathbf{1 . 7 8 6 3 \times 1 0 ^ { - \mathbf { 4 } } \boldsymbol { M }}\end{gathered}$$\)
The solubility equilibrium is given by:
\($$\mathrm{Ce}\left(\mathrm{IO}_{3}\right)_{4(s)} \rightleftharpoons \mathrm{Ce}_{(a q)}^{3+}+3 \mathrm{IO}_{3}^{-}(a q)$$\)
Therefore, the solubility product is given by:
\(\begin{gathered}K_{s p}=\left[C e^{3+}\right] \cdot\left[I O_{3}^{-}\right]^{3} \\\therefore K_{s p}=\left(1.7863 \times 10^{-4}\right) \times\left(3 \times 1.7863 \times 10^{-4}\right)^{3}\end{gathered}\)
\(\therefore {K_{s p}=2.749 \times 10^{-14}\)
Some advocates of anabolic steroid use report that testicular atrophy associated with use of trenbolone can be alleviated if users also inject themselves with human chorionic gonadotropin, a hormone similar in structure and function to LH and FSH. Please explain how this treatment might work.
Answers
Answer:
Human chorionic gonadotropin (HCG) injections are much safer to use with some mild side effects to treat issues of testicular atrophy. Human chorionic gonadotropin (HCG) injections would increase blood flow and enable the testes' produce testosterone, reduce the shrinkage of the testicles and it also aids in the production of sperm cells which has been initially impaired due to testicular atrophy.
Explanation:
Anabolic steroid is a drug that plays the role of testosterone.
Testicular atrophy is the shrinkage of the testicles caused by old age or infections. Once it occurs, it lowers the production of testosterone and sperm cells.
Trenbolone increases muscle building, aids lean fat deposition, reduces the production of testosterone, and could also lead to testicular atrophy.
How does a Sulfur 2 ion differ from a neutral Sulfur atom?
O
Mass number
Atomic number
Number of electrons
Proton number
Answers
Answer:
o
Mass number
Atomic number
Number of electrons
Proton number
Explanation:
1Li3 + 3H2O -> 1NH3 + 3LiOH
Determine the mass of lithium hydroxide produced when 0.38g of lithium nitride reacts with an excess of water.
Answers
0.785 g of lithium hydroxide (LiOH) are produced when 0.38 g of lithium nitride (Li₃N) reacts with an excess of water.
we can see that 3 moles of lithium hydroxide (LiOH) are produced for every 1 mole of lithium nitride (Li₃N) that reacts.
To determine the mass of LiOH produced from 0.38 g of Li₃N, we need to first calculate the number of moles of Li₃N present:
molar mass of Li₃N = 3 x atomic mass of Li + 1 x atomic mass of N
= 3 x 6.94 g/mol + 1 x 14.01 g/mol
= 34.83 g/mol
moles of Li₃N = mass / molar mass
= 0.38 g / 34.83 g/mol
= 0.01093 mol
Since the balanced equation shows that 1 mole of Li₃N produces 3 moles of LiOH, we can calculate the number of moles of LiOH produced:
moles of LiOH = 3 x moles of Li₃N
= 3 x 0.01093 mol
= 0.03279 mol
Finally, we can use the molar mass of LiOH to convert from moles to grams:
molar mass of LiOH = atomic mass of Li + 1 x atomic mass of O + 1 x atomic mass of H
= 6.94 g/mol + 15.99 g/mol + 1.01 g/mol
= 23.94 g/mol
mass of LiOH produced = moles of LiOH x molar mass of LiOH
= 0.03279 mol x 23.94 g/mol
= 0.785 g
Therefore, approximately 0.785 g of lithium hydroxide (LiOH) are produced when 0.38 g of lithium nitride (Li₃N) reacts with an excess of water.
learn more about mass here
https://brainly.com/question/837939
#SPJ1
milk has a pH of 6.0 and household ammonia has a pH of 12.0. How much more acidic is milk than ammonia
Answers
60 I think bcz if there is 1&2 they differ 10 times
The milk has a pH of 6.0 and household ammonia has a pH of 12.0. then milk will be 6 times milk acidic than ammonia.
What is pH?The pH scale, which previously stood for "potential of hydrogen," would be used to describe how acidic or basic an aqueous solution is.
What is an acidic solution?More hydrogen ions are present in an acidic solution than it was in pure water.
It is given then,
\((milk)pH_{1} = 6\\(ammonia)pH_{2} = 12\)
The concentration of hydrogen ions can be calculated by using the formula:
\([H^{+}] = 10^{-pH}\)
By putting the value of given data in the above equation.
\([H^{+}_{milk} ] / H^{+}_{ammonia}= 10^{-6}/10^{-12}\\ = 10^{6} = 6\)
We get that, milk will be 6 times more acidic than ammonia.
To know more about pH and acidic solutions
https://brainly.com/question/15289741
#SPJ2
The symbol that indicates a substance dissolve in water is
Answers
The symbol that indicates a substance dissolve in water is (aq).
What is the symbol?The word "(aq)" stands for aqueous and denotes a material that dissolves in water.
Aqueous solutions are created when substances dissolve in water and are uniformly dispersed throughout the liquid. "(aq)" is added to the end of a substance's chemical formula in a chemical equation to denote that the material is in an aqueous state.
We often see this in several chemical reactions and the symbols shows that the solute was dissolved in water.
Learn more about soluble in water:https://brainly.com/question/10523710
#SPJ1
Explain the difference between an ideal and a nonideal solution.
Answers
Answer:
The difference between an ideal and a nonideal solution is given below:-
Explanation:
Ideal Solution:
The ideal solution is a method where the relationships of all the molecules in the mixture are similar. Upon combining it with a solvent, the distance between the solute molecules does not increase. It is because for increasing the distance, there should also be a force that acts on everyone and every molecule of the solute mixture.Non-ideal Solution:
The non-ideal aqueous solution that has distinctions in the system provides particles of different sizes of different components. The power of the molecular interactions can be identified as a non-ideal solution.How many mEq are in 4.5L of 0.8M MgCl2
Answers
Explanation:
We have to find the number of mEq in 4.5 L of 0.8 M solution of MgCl₂. First we can find the number of moles of MgCl₂ that are present in that solution.
The molarity can be calculate using the following formula.
molarity of a solution = number of moles of solute/(volume of solution in L)
We already know the concentration of the solution and the volume of solution in liters. So we can find the number of moles of the salt that are present in the solution.
molarity of a solution = moles of MgCl₂/(volume of solution in L)
moles of MgCl₂ = molarity of the solution * volume of solution in L
moles of MgCl₂ = 0.8 M * 4.5 L
moles of MgCl₂ = 3.6 mol
So we found that there are 3.6 mol of MgCl₂ in the solution. Now we have to find the number of Eq or mEq.
The equivalent mass of a salt can be defined like the molecular mass of the salt divided by the charge of the metal cation.
MgCl₂ ---> Mg²⁺ + 2 Cl⁻
The atom of Mg is transferring one electron to each atom of Cl. That means that it is losing two electrons.
Equivalent mass of MgCl₂ = molar mass of MgCl₂/2
That means that each mol of MgCl₂ contains two equivalents.
Equivalents of MgCl₂ = 2 * number of moles of MgCl₂
Equivalents of MgCl₂ = 2 * 3.6 Eq
Equivalents of MgCl₂ = 7.2 Eq = 7.2 Eq * 1000 mEq/Eq
Equivalents of MgCl₂ = 7200 mEq
Answer: There are 7200 mEq of MgCl₂ in the solution.
Is it possible for number of moles to be less than one?
Answers
Answer:
yes is very possible to be
Which letter indicates the asthenosphere
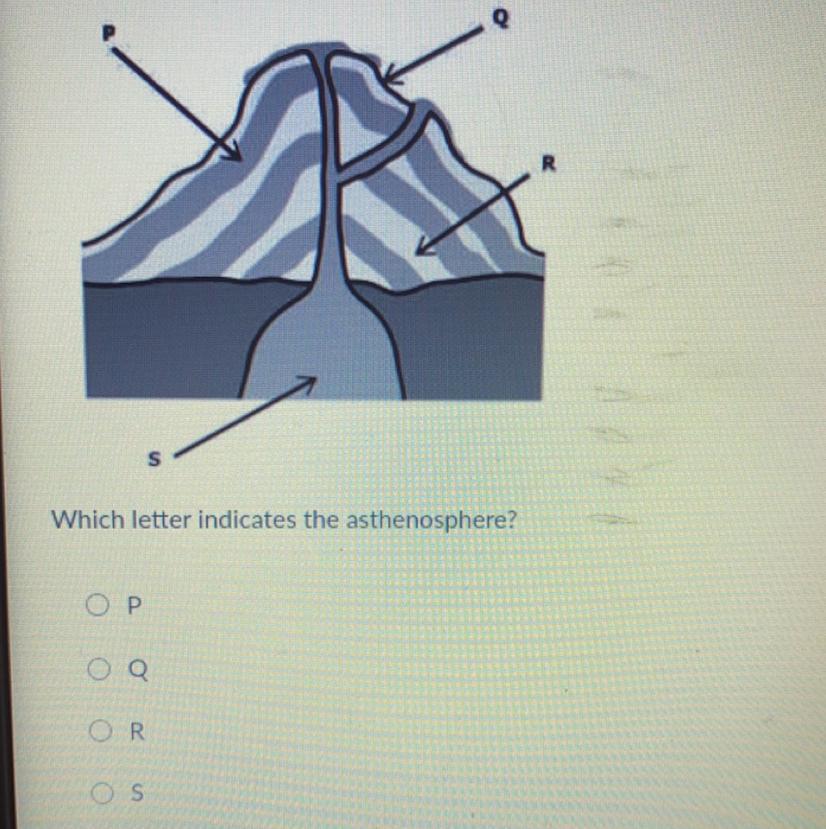
Answers
Answer:
q
Explanation:
Chlorine gas reacts with fluorine gas to form chlorine trifluoride. Cl2(g)+3F2(g)→2ClF3(g) A 2.05 L reaction vessel, initially at 298 K, contains chlorine gas at a partial pressure of 337 mmHg and fluorine gas at a partial pressure of 730 mmHg .
Answers
Answer:
2.4 grams of ClF3
Explanation:
First let us determine the moles of Cl2 and F2,
Cl2 = ( ( 337 )( 2.05 L ) / ( 0.082 )( 298 K ) ) * ( 1 atm / 780 ),
Cl2 = ( 690 / 24.436 ) * ( 1 / 780 ),
Cl2 = ( About ) 0.036 moles of Cl2
_________________________________________________
F2 = ( ( 729 )( 2 L ) / ( 0.082 )( 298 K ) ) * ( 1 atm / 780 ),
F2 = ( 1458 / 24.436 ) * ( 1 / 780 )
F2 = ( About ) 0.078 moles of F2
Now let us identify the limiting reactant, considering the ratio between ClF3 and Cl2 / F2. In this case F2 is the limiting reactant, as it forms a smaller molar ratio;
The theoretic yield is thus performed with the limiting reactant F2,
0.078 * ( 2 / 3 ) * ( 92.45 / 2 ) = ( About ) 2.4 grams of ClF3
Help!!!
Question: What is the correct order of the particles that give texture to soil from smallest to largest?
Options:
A: Clay, sand, silt
B: silt, sand, clay
C: clay, silt, sand
D: sand, silt, clay
Answers
The correct order of the particles that give texture to the soil from smallest to largest is clay, silt, and sand; option C.
What is soil texture?Soil texture refers to a physical classification of the component and types of soils based on their physical texture either as coarse or fine particles.
There are several types of soils and these various types of soils have different textures.
The types of soils and their arrangement based on increasing particle size are as follows:
clay soil - this is the finest particle soil typesilt - this is the next soil type in terms of texturesandy soil - this is the largest of the three soil types in terms of size and texture.Learn more about soil texture at: https://brainly.com/question/8513717
#SPJ1
Select all answer choices that would result in units of moles.
a) RT/PV
b) PV/RT
c) mass ÷ molar mass
d) molar mass ÷ mass
e) molar mass × mass
f) molarity ÷ volume
g) volume ÷ molarity
h) molarity × volume
Answers
The correct options that will result in mole are option B, C, and H
How do i know which options will result in mole?To know the options that will result in mole, do the following:
Ideal gas law states as follow:
PV = nRT
Where
P is the pressure V is the volumen is the number of moleR is the gas constantT is the temperaturePV = nRT
Make n the subject by dividing both sides by RT
n = PV / RT (option B)
Mole, mass and molar mass are related according to the following formula:
Mole = mass / molar mass (option C)
Molarity is defined as mole per unit volume as shown below:
Molarity = mole / volume
Make mole the subject by cross multiplying.
Mole = Molarity × volume (option H)
Thus, from the above illustrations, we can conclude that the correct options which will result in mole are option B, C, and H
Learn more about mole:
https://brainly.com/question/30337259
#SPJ1
Find the mole fraction of Methanol CH3OH and water in a solution prepared by dissolving 4.5 g of alcohol in 40 g of H2O.
Answers
How could a solid result from the mixing of two liquids?
Answers
Answer:
Combining the two clear colorless liquids is a chemical change because a different solid substance is formed. Tell students that a precipitate is an insoluble solid that forms when two solutions are combined and react chemically. Insoluble means that the solid will not dissolve.
Explanation:
PLEASE HELP THIS IS IMPORTANT!!! I WILL MARK BRAINLIEST
Which plate boundary and movement commonly create volcanoes that can result in the development of habitable islands? Explain how islands can be created by plate tectonics.
PLEASE MAKE ANSWER SIMPLE AND I WILL GIVE LOTS OF POINTS AND MARK BRAINLIEST
Answers
Answer:Which plate boundary and movement commonly create volcanoes that can result in the development of habitable islands?
Sometimes, the plates collide with one another or move apart. Volcanoes are most common in these geologically active boundaries. The two types of plate boundaries that are most likely to produce volcanic activity are divergent plate boundaries and convergent plate boundaries
Explain how islands can be created by plate tectonics.
Explanation: These hot spots are able to independently melt the tectonic plate above them, creating magma that erupts onto the top of the plate. In hot spots beneath the ocean, the tectonic activity creates a volcanic mound. Over millions of years, volcanic mounds can grow until they reach sea level and create a volcanic island.
The radius of a single atom of a generic element X is 151 picometers (pm) and a crystal of X has a unit cell that is body-centered cubic. Calculate the volume of the unit cell.
Answers
The volume of the unit cell of element X is approximately 1.785 × 10⁸ picometers cubed (pm³).
What is the volume of the unit cell?
In a body-centered cubic unit cell, there are atoms at each of the eight corners of the cube, and one atom in the center of the cube. Each atom at the corner is shared by eight unit cells, while the atom at the center is contained within a single unit cell.
The distance from one corner of the cube to the opposite corner is equal to four times the radius of the atom (i.e. 4 × 151 pm = 604 pm), which is known as the body diagonal of the cube.
Therefore, the length of one side of the cube is given by:
a = (4/√3) × r
= (4/√3) × 151 pm
≈ 554.6 pm
The volume of the unit cell is then given by:
V = a³
= (554.6 pm)³
≈ 1.785 × 10⁸ pm³
Learn more about volume of unit cell here: https://brainly.com/question/28191873
#SPJ1
WHEN SOME PEOPLE HAVE AN UPSET STOMACH, THEY TAKE A SODA TABLET LIKE
TUMS TO NEUTRALIZE THEIR STOMACH ACID.
THE REACTION IS HYDROCHLORIC ACID PLUS SODIUM BICARBONATE MAKES SALT,
CARBON DIOXIDE (THAT'S WHY SOME PEOPLE BURP) AND WATER.
HOW MUCH CARBON DIOXIDE AND SALT (IN GRAMS) ARE PRODUCED IF A 2 GRAM
TABLET OF SODIUM BICARBONATE IS TAKEN TO REACT WITH 18 GRAMS OF
HYDROCHLORIC ACID?
Answers
The balanced chemical equation for the reaction between hydrochloric acid (HCl) and sodium bicarbonate \((NaHCO_3)\) is:
\(HCl + NaHCO_3\ - > NaCl + CO_2 + H_2O\)
The coefficients in the balanced equation show that 1 mole of HCl reacts with 1 mole of \(NaHCO_3\) to produce 1 mole of NaCl, 1 mole of \(CO_2\), and 1 mole of \(H_2O\). We need to find the number of moles of \((NaHCO_3)\) present in the tablet.
2 grams of \(NaHCO_3\) is equivalent to 0.02 moles, and 18 grams of HCl is equivalent to 0.45 moles. Since \((NaHCO_3)\) is limiting reagent, only 0.02 moles of NaCl and \(CO_2\) will be produced. The molar mass of \(CO_2\) is 44 g/mol, so the mass of \(CO_2\) produced is 0.88 g. The molar mass of NaCl is 58.44 g/mol, mass of NaCl produced is 1.17 g.
To know more about hydrochloric acid, here
brainly.com/question/15231576
#SPJ1
10-kg of R-134a at 300 kPa fills a rigid container whose volume is 14 L. Determine the temperature and total enthalpy in the container. The container is now heated until the pressure is 600 kPa. Determine the temperature and total enthalpy when the heating is completed.
Answers
Answer:
Temperature = 0.605°C
Total enthalpy at 300kpa = 545.2 kJ
Total enthalpy at 600kpa = 846.45 kJ.
Explanation:
Checking the table for 134a pressure table. It is given that the specific volume of saturated liquid and the specific volume of the saturated vapor of 280kpa is 0.0007699 m^3/kg and 0.072352 m^3/kg respectively.
Also, the specific volume of saturated liquid and the specific volume of the saturated vapor of 320kpa is 0.0007772 m^3/kg and 0.063604 m^3/kg respectively.
The first thing to do is to determine the value for the specific volume of saturated liquid.
At 300 kpa, the specific volume of saturated liquid,n is given below as;
300 - 280/ 320 - 280 = (n - 0.0007699)/ 0.0007772 - 0.0007699.
Therefore, n = specific volume of saturated liquid = 0.0007735 m^3/kg.
300 - 280/ 320 - 280 = n - 0.072352/ (0.063604 - 0.072352).
n = 0.0679 m^3/kg.
The second thing to do is to determine the value of the specific volume.
Specific volume = 14 × 10^-3/ 10 = 0.0014 m^3/kg.
Determine the enthalpy of the mixture,b(I). This is given below as;
300 - 280/ 320 - 280 = b(I) - 199.54/ (196.7 - 199.54).
b(I) = 198.125 kJ/Kg.
Hence, b = [ 300 - 280/ 320 - 280 = j - 50.18 / 55.16 - 50.18] + [ ( 0.0014 - 0.00077735) / 0.067978 - 0.00077735] × 198.125.
b = 54.517 KJ/Kg.
Total enthalpy = 10 × 54.517 = 545.17 kJ.
Temperature can be Determine as below;
300 - 280/ 320 - 280 = T + 1.25 / 2.46 - 1.25.
Temperature = 0.605°C.
Hence, at 600kpa, the total enthalpy = [81.51 + ( 0.0014 - 0.0008199/ 0.034295 - 0.0008199) × 180.90] × 10
Total enthalpy at 600kpa = 846.45 kJ.
A compound is 22.5% nitrogen and 77.5% oxygen, What is the empirical formula of
this compound?
Answers
Answer:
\(NO_3\)
Explanation:
Hello!
In this case, since percent compositions are used to set up empirical formulas when assuming those percentages are masses, we can fist compute the moles of nitrogen and oxygen in the compound as shown below:
\(n_N=22.5g/14.01g/mol=1.61mol\\\\n_O=77.5g/16.00g/mol=4.84mol\)
Now, we divide by the fewest moles to compute the subscripts:
\(N=\frac{1.61}{1.61} =1\\\\O=\frac{4.84}{1.61} =3\)
Thus, the empirical formula turns out:
\(NO_3\)
Best regards!
what is the photoelctric effect?
Answers
Explanation:
It is the emission of electron from a metal under the effect of light is known as photo electric effect
I hope this imformation help full for you
2. What is the pH of the following solutions?
2.1. 50 mmol.dm solution of Ba(OH)2
Answers
i hope this helps (please mark brainliest)

What is the molar mass
MgCrO4
Answers
The molar mass of MgCrO4 is approximately 140.30 g/mol.
To determine the molar mass of MgCrO4 (magnesium chromate), we need to calculate the sum of the atomic masses of each individual element in the compound.
The chemical formula MgCrO4 indicates that the compound consists of one magnesium atom (Mg), one chromium atom (Cr), and four oxygen atoms (O).
The atomic masses of the elements can be found on the periodic table:
Magnesium (Mg) has an atomic mass of approximately 24.31 g/mol.
Chromium (Cr) has an atomic mass of around 51.99 g/mol.
Oxygen (O) has an atomic mass of about 16.00 g/mol.
Now, we can calculate the molar mass of MgCrO4 by summing up the atomic masses of each element, considering the respective subscripts:
Molar mass = (Atomic mass of Mg) + (Atomic mass of Cr) + 4 × (Atomic mass of O)
Molar mass = (24.31 g/mol) + (51.99 g/mol) + 4 × (16.00 g/mol)
Molar mass = 24.31 g/mol + 51.99 g/mol + 64.00 g/mol
Molar mass ≈ 140.30 g/mol
for more such questions on mass
https://brainly.com/question/24191825
#SPJ8
What type of intermolecular force will for between H2O AND CH3OH? Draw and label a picture of this bond. Explain in words how this bond forms.

Answers
Hydrogen bonding, which is unquestionably what we have, will occur from the intermolecular force between the molecules of H2O and CH3OH. Atoms trade or exchange valence electrons to create bonds.
How come we create bonds?Trust and self-esteem are developed in children and adolescents through strong emotional ties. After that, they can leave the family and establish wholesome friendships and other types of social ties. Healthy relationships consequently lower a child's chances of emotional discomfort or antisocial behaviour.
What exactly is a bonds, for example?The government of a country issues government bonds, a sort of fixed-interest bond. These bonds are thought of as low-risk investments. Examples of different kinds of government bonds include T - bills, Municipality Bond, Zero-Coupon Bonds, and others.
To know more about intermolecular visit:
https://brainly.com/question/9007693
#SPJ1
If the 50 kg object slows down to velocity of 1 m\s how much Kinect energy dose it have
Answers
So 1/2*50*1= 25Joules
The complex ion Cu(NH3)42+ is formed in a solution made of 0.0200 M Cu(NO3)2 and 0.300 M NH3. What are the concentrations of Cu2+, NH3, and Cu(NH3)42+ at equilibrium? The formation constant*, Kf, of Cu(NH3)42+ is 1.70 × 1013.
Answers
The concentrations Cu(NH3)42+ at equilibrium is [Cu(NH3)42+] = 1.70 × 1013 * (0.0200M) * (0.300M)^4.
The concentrations of Cu2+ is [Cu(NH3)42+] + [Cu2+]
The concentrations of NH3 is 4[Cu(NH3)42+] + 4[NH3]
What is concentration equilibrium?Equilibrium concentration is described as a state when the rate of forward reaction in a chemical reaction becomes equal to the rate of backward reaction.
The equilibrium constant expression for the formation of the complex ion Cu(NH3)42+ is:
Kf = [Cu(NH3)42+] / [Cu2+] * [NH3]^4
where [Cu(NH3)42+], [Cu2+], and [NH3] are the molar concentrations at equilibrium.
The initial concentrations of Cu2+ and NH3 are 0.0200 M and 0.300 M respectively.
We have that Kf = 1.70 × 1013, we then rearrange the equation to solve for [Cu(NH3)42+]:
1.70 × 1013 = [Cu(NH3)42+] / (0.0200M) * (0.300M)^4
[Cu(NH3)42+] = 1.70 × 1013 * (0.0200M) * (0.300M)^4
Therefore at equilibrium, the concentration of Cu(NH3)42+ is [Cu(NH3)42+] = 1.70 × 1013 * (0.0200M) * (0.300M)^4
Learn more about Equilibrium concentration at: https://brainly.com/question/13414142
#SPJ1
A chemist uses 22.0 mL of 0.10 M H₂SO4 to neutralize 10.0 mL of NaOH. What is the concentration of the NaOH solution?
2 A 044M
OB. 0.22 M
O C 0.11 M
OD. 0.055 M
Answers
The concentration of the NaOH solution required for the reaction given the data is 0.44 M
Balanced equationH₂SO₄ + 2NaOH —> Na₂SO₄ + 2H₂O
From the balanced equation above,
The mole ratio of the acid, H₂SO₄ (nA) = 1The mole ratio of the base, NaOH (nB) = 2How to determine the concentration of NaOHVolume of acid, H₂SO₄ (Va) = 22 mL Concentration of acid, H₂SO₄ (Ca) = 0.1 MVolume of base, NaOH (Vb) = 10 mLConcentration of base, NaOH (Cb) =?CaVa / CbVb = nA / nB
(0.1 × 22) / (Cb × 10) = 1 / 2
2.2 / (Cb × 10) = 1 / 2
Cross multiply
Cb × 10 = 2.2 × 2
Cb × 10 = 4.4
Divide both side by 10
Cb = 4.4 / 10
Cb = 0.44 M
Thus, the concentration of the NaOH solution is 0.44 M
Learn more about titration:
https://brainly.com/question/14356286
#SPJ1
Need actual help plsss

Answers
5. Which organelle, found in plant and
animals cells, can store wastes and
protect the cell from contamination?