Write the common name of the α‑ketoacid resulting from the transamination of each amino acid.
Answers
The α‑ketoacid or the α‑ carboxylic acid are the organic compounds that are the characterize the presence of a keto group at the position of α- in carboxylic acid functional group.
The common name of the α‑ketoacid resulting from the transamination of each amino acid is given as follows :
1) alanine + α‑ketoglutarate ---> pyruvate
2) aspartate + α‑ketoglutarate ----> oxaloacetate
3) glutamate + α‑ketoglutarate -----> α‑ketoglutarate
4) phenylalanine + α‑ketoglutarate ------> phenylpyruvate
5) tyrosine + α‑ketoglutarate -----> p - hydroxyphenyl pyruvate
The amino acids are the organic compounds which contain both the groups the amino acid and the carboxylic acid groups.
To learn more about amino acid here
https://brainly.com/question/14171266
#SPJ4
Related Questions
Two substances were combined and immediately a gas was produced. What type of change is happening?.
Answers
Answer: This is called a chemical reaction.
Explanation: Gas may form, heat may be produced, and color may change. These types of changes indicate that a chemical reaction has occurred.
Hi! Can you help me?
Is NaCI an element, compound, or mixture?
Thanks!
Answers
Answer:
Compound
Explanation:
Sodium chloride, commonly known as salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl
"Reaction mechanisms usually involve only unimolecular or bimolecular steps."
Is this statement true or false?
Answers
" Reaction mechanisms usually involve only unimolecular or bimolecular steps". This statement is true.
A reaction mechanism involves only unimolecular and/or bimolecular elementary steps because in higher reactions all three or more reactants have to collide at the same time which is most likely to not happen.
Unimolecular reactions are mostly of first order. It occurs when a molecule rearranges itself to give one or more products in a reaction. For example, in radioactive decay, some particles are emitted from the atom giving different atoms in the reaction. In bimolecular reaction, two of the molecules collide with each other to give one or more products. Mostly occur in organic reactions for example, nucleophilic substitution reaction.
In higher reactions such as termolecular reactions unlike unimolecular and bimolecular reactions, all three or more reactants should collide with same energy and proper orientation to give products which is most likely to not take place.
To know more about reaction mechanism here
https://brainly.com/question/3082927
#SPJ4
which component of a chemical reaction does not change from beginning to end?
Answers
The component of a chemical reaction that does not change from beginning to end is the mass. According to the law of conservation of mass, matter can neither be created nor destroyed in a chemical reaction.
The total mass of the reactants before the reaction must be equal to the total mass of the products after the reaction. This means that the mass is conserved throughout the reaction. Therefore, the mass is the component of a chemical reaction that does not change from beginning to end.
However, while the mass of the substances remains the same, their chemical composition does change due to the rearrangement of atoms and formation of new chemical bonds.
To know more about chemical visit-
https://brainly.com/question/29240183
#SPJ11
Which subatomic particle has a positive charge?
Question 1 options:
all particles
neutron (n0)
proton (p+)
electron (e-)
Question 2 (2 points)
What information does the atomic mass of an element provide?
Question 2 options:
the difference between the number of protons and electrons (p - e)
the number of isotopes
the sum of electrons and protons in an atom (e + p)
the number of protons and neutrons in an atom (p + n)
Question 3 (2 points)
What is the atomic number of an atom?
Question 3 options:
the number protons and neutrons
the number of protons
the number of neutrons
the number of electrons and neutrons
Question 4 (2 points)
What is H2O (water) classified as?
Question 4 options:
a diatomic molecule
an atom
an element
a molecule
Question 5 (2 points)
Which property is a physical property?
Question 5 options:
easy to digest
becomes moldy quickly
does not burn
malleable (the quality of something that can be shaped into something else without breaking )
Question 6 (2 points)
What state of matter has a defined volume but undefined shape?
Question 6 options:
solids and liquids
liquids
liquids and gases
gases
Question 7 (2 points)
Which statement best describes the difference between a physical change and a chemical change?
Question 7 options:
A chemical change does not result in a new substance being formed, whereas a physical change does.
A chemical change results in a new element being formed, and a physical change results in a new compound being formed.
A physical change produces a new element, and a chemical change results in a bond breaking between atoms.
A physical change is a change from one state of matter to another; a chemical change results in a new substance formed.
Question 8 (2 points)
Where are nonmetals located in the periodic table?
Question 8 options:
in the middle
along the bottom
along the upper left side
along the upper right side
Question 9 (2 points)
The elements fluorine (F), chlorine (Cl), and Iodine are all part of the same ____________________ on the periodic table.
Question 9 options:
period
group
Question 10 (2 points)
How are elements arranged on the periodic table?
Question 10 options:
atomic mass (# of protons and neutrons)
atomic number (# of protons)
density
boiling point
Help please ill give 100 points
Answers
Answer:
SCORE WARNING ! ! ! (JUST FINISHED MY TEST AND GOT A B IM NOT SURE WHICH ONE WAS INCORRECT btw it was the K12 8th grade test)
1. C / Proton (p+)
2. D / the number of protons and neutrons in an atom (p + n)
3. B / the number of protons
4. D / a molecule
5. B / becomes moldy quickly
6. D / gases
7. D / A physical change is a change from one state of matter to another; a chemical change results in a new substance formed.
8. D / upper right side
9. B / group
10. B / atomic number (# of protons)
Explanation:
I just took the test
The positively charged subatomic particles are called protons. The mass number of an atom is the sum of number of protons and neutrons and the atomic number is the number of protons.
What is an atom?An atom is the basic unit every substances. It is made up of subatomic particles electrons, protons and neutrons. The positively charged particles are called protons. Whereas electrons are negatively charged.
The atomic number is the number of protons and the mass number indicates the sum of number of protons and neutrons.
Water can be classified as a diatomic molecule formed from two hydrogens and one oxygen atom. Among the given, malleability is a physical property. It is the ability to change the shape and size of metal blocks.
Liquids has definite volume but does not have a defined shape. They assume shape of the container.A physical change is a change from one state of matter to another; a chemical change results in a new substance formed.
Nonmetals are located in the side upper right side of periodic table. Metals are covered in left and middle portion. The elements F, Cl are in same group. All the elements are arranged in periodic table based on their atomic number.
To find more on periodic table refer here:
https://brainly.com/question/11155928
#SPJ2
Please help
urgently!

Answers
1. We can see here that energy is required to change the phase of matter. For example, energy is required to melt ice, vaporize water, and condense steam. The amount of energy required to change the phase of matter is called the latent heat.
What is energy?Energy is a fundamental concept in physics and refers to the ability or capacity of a system to do work or produce a change.
2. The demonstration on the sample of water showed that water can exist in three phases: solid, liquid, and gas. The solid phase is ice, the liquid phase is water, and the gas phase is steam.
The demonstration started with ice at 0°C. As heat was added to the ice, the temperature of the ice increased. However, the ice did not melt until the temperature reached 0°C. This is because the energy from the heat was used to break the bonds between the water molecules in the ice. Once the bonds were broken, the ice melted and became water.
3. Completing the
When all the intermolecular bonds are overcome, the transition between phases is complete. The energy of any substance includes the kinetic energy, potential energy, and thermal energy of its particles.Page 4:
Heating and cooling curves are graphical representations of how temperature changes during the process of heating or cooling a substance. They illustrate the relationship between temperature and the state of matter.
Heating curves represent the temperature changes of a substance as it is heated.Cooling curves, on the other hand, represent the temperature changes of a substance as it is cooled.Both curves show:
Plateaus or flat sections: These occur during phase transitions where the temperature remains constant despite the addition or removal of heat.Changes in slope: The slope of the curve represents the rate of temperature change. Steeper slopes indicate faster changes in temperature, while shallower slopes indicate slower changes.Learn more about energy on https://brainly.com/question/2003548
#SPJ1
9. What is the process of nuclear fusion? *
(10 Points)
O A. The process of carrying light
B. The process of two atoms fusing
O C. The process of gravity and inertia keeping the universe together
O D. The process of fusing two stars
Answers
Answer:
the process of carrying light
If you have a hydrocarbon with 17 carbons, how many hydrogens will it contain to be fully saturated?
Answers
If you have a hydrocarbon with 17 carbons, it will take 34 hydrogens to be fully saturated.
What is hydrocarbon?A hydrocarbon is an organic molecule composed completely of hydrogen and carbon in organic chemistry. Group 14 hydrides include hydrocarbons. Hydrocarbons are often colorless and hydrophobic, with scents that are weak or exemplified by gasoline and lighter fluid.
They exist in a wide range of molecular forms and phases, including gases (like methane and propane), liquids (like hexane and benzene), low melting solids (like paraffin wax and naphthalene), and polymers (such as polyethylene and polystyrene). Hydrocarbon refers to naturally occurring petroleum, natural gas, and coal, as well as their hydrocarbon derivatives and refined forms, in the fossil fuel industry. The primary source of energy on the planet is the combustion of hydrocarbons.
To learn more about hydrocarbons visit:
https://brainly.com/question/14049901
#SPJ4
Some analytes must be derivatized to increase their column retention or detectability. Derivatization means Group of answer choices altering the chemical structure of the analyte to increase detection and specificity. adding fluorescent labels or combining the analyte with chiral reagents or other chemicals to increase detectability. removing dissolved gases in the solvent to produce a clear chromatogram. using multiple detectors to assist in identification.
Answers
Derivatization means adding fluorescent labels or combining the analyte with chiral reagents or other chemicals to increase detectability.
Some analytes must be derivatized to increase their column retention or detectability.
Retention time can be referred to as the amount of time a solute spends in the stationary and mobile phases of a column.
Detectability is the ability of an analyte to get detected in the mobile phase of chromatography.
The refractometer, fluorescence detector, and UV detector are the three most popular liquid chromatography detectors. These detectors increase the detectability.
For derivatization, the fluorescence detector are used.
Learn more about derivatization
https://brainly.com/question/17609332
#SPJ4
HI PLEASE HALP MEH
Habitat preservation is usually the most efficient method of protecting biodiversity because it involves protecting an entire ____.
A: Organism
B; population
C: ecosystem
D: species
Answers
Habitat preservation is usually the most efficient method of protecting biodiversity because it involves protecting an entire ecosystem.
What are natural habitats?The natural world provides a variety of resources that can be taken advantage of for financial gain, such as timber taken from forests and clean water taken from streams and hence the entire ecosystems preservation.
However, anthropogenic economic growth-related land expansion frequently results in a decrease in the ecological integrity of neighboring natural habitat.
A management strategy known as habitat conservation aims to preserve, maintain, and restore habitats in order to stop the extinction, fragmentation, or range reduction of species. It is a focus of numerous groups that is difficult to categorize in terms of a single ideology.
For instance in tropical regions with a high species richness or in leisure activities like hiking and mountain biking that take place in undeveloped areas. Repairing harmed ecosystems is thought to be substantially more valued.
To find more on ecosystems, refer here:
https://brainly.com/question/13979184
#SPJ6
The structure and bonding of diamond, which is formed from graphite at extreme pressures, should be similar to that of elemental:
Answers
Answer:
silicon and germanium.
Explanation :
The passage states that at extreme pressures an elemental solid assumes the structure and bonding characteristics of a heavier element in the same column of the periodic table. The structure and bonding of diamond, which is a form of carbon, would therefore be most like other elements in the same group of the periodic table as carbon. This group contains silicon and germanium.
How many grams of water should be added to 4.00 g NaOH to create 2.00% by mass NaOH solution?
Answers
Answer:
196 grams
because ^^^ was wrong and person in comments said it was 196 and it was right
When NaOH of 4.00 g is being added with water, the mass of water required to create 2.00% of NaOH solution by mass is 196 g.
What is mass percent of solute?It means that the particular amount of solute in terms of percentage is present in the solute.
Given the mass of the solute, NaOH is 4.00g
Suppose the mass of water required to add is x in g.
The mass percent of the solute is
\(\dfrac{2}{100} =\dfrac{4.00}{4.00 +x }\)
x =196 g
Therefore, mass of water required to create 2.00% of NaOH solution by mass is 196 g.
Learn more about Mass percent of solute.
https://brainly.com/question/15136748
#SPJ2
Given the following reaction:
H2(g) + I2(g) <---> 2HI(g) Kc = 57.85
1.50 mol of each reactant was placed in a 2.00-L flask.
At equilibrium what is the concentration of HI?
At equilibrium what is the concentration of H2 and I2?
Answers
The equilibrium concentration of H2 and I2 is 0.165 M. The equilibrium concentration of HI is 1.17 M.
What is Kc?The term Kc refers to the equilibrium constant for a aqueous phase reaction. The concentrations of each specie is;
H2 = 1.5/2 = 0.75 M
I2 = 1.5/2 = 0.75 M
Setting up the ICE table;
H2(g) + I2(g) <---> 2HI(g)
I 0.75 0.75 0
C -x -x +2x
E 0.75 - x 0.75 - x 2x
Kc = [HI]^2/[H2] [I2]
57.85 = (2x)^2/(0.75 - x)^2
57.85(0.56 - 1.5x + x^2) = 4x^2
32.3 - 86.7x + 57.85x^2 = 4x^2
4x^2 - 57.85x^2 + 86.7x - 32.3 = 0
-53.85x^2 + 86.7x - 32.3 = 0
x = 0.585 M because x can not be greater than the initial concentration.
At equilibrium;
[HI] = 2x = 2(0.585) = 1.17 M
[H2] =[I2] = 0.75 M - 0.585 M = 0.165 M
Learn more about equilibrium: https://brainly.com/question/17960050
Give the moles of vanillin reducible by 0.5 mole sodium borohydride.
Answers
Although it is soluble in water, sodium borohydride interacts with water. Hydrogen gas is created as a reaction byproduct when hydrogen atoms are dissolved in water. Gaseous hydrogen is very flammable.
What is the properties of sodium borohydride?The powder form of sodium borohydride is white to grey. Water breaks it down into hydrogen, a combustible gas, and sodium hydroxide, a caustic substance.
This process could generate enough heat to ignite the hydrogen. The substance itself ignites quickly and burns intensely once it does.
A white, odorless powder or pellet is sodium borhydride. It serves as a reducing agent for aldehydes and ketones, a blowing agent for polymers, and a bleaching agent for wood pulp.
Therefore, Aldehydes can be preferentially reduced in the presence of other functional groups, such as ketones, esters, imides, and nitriles, due to NaBH4's high reactivity to the carbonyl carbon's positive charge.
Learn more about sodium borohydride here:
https://brainly.com/question/28633622
#SPJ1
In which of the following phenomena do free electrons play a role? O Thermal expansion O Thermal conduction
Answers
Free electrons play a role in thermal conduction. Thermal conduction is the transfer of heat energy through a material or between materials in direct contact. In metals, which are good conductors of heat, free electrons contribute significantly to the process of thermal conduction.
In metals, some of the electrons in the outer energy levels of atoms are not tightly bound to individual atoms but are relatively free to move throughout the material. These free electrons are often referred to as conduction electrons. When there is a temperature gradient in a metal, the free electrons gain kinetic energy from the higher-temperature region and transfer it to neighboring atoms by colliding with them. This transfer of kinetic energy is what facilitates the conduction of heat through the material.
On the other hand, thermal expansion refers to the expansion or contraction of a material due to changes in temperature. While free electrons are present in metals, they do not play a direct role in the phenomenon of thermal expansion. Thermal expansion is primarily determined by the behavior of atoms or molecules within the material.
Therefore, free electrons are specifically involved in thermal conduction rather than thermal expansion.
To know more about atoms
brainly.com/question/1566330
#SPJ11
what is the mass percent of hydrogen in water
Answers
Answer:
11.11%
Explanation:
The percentage of an element in a compound is 100 times the fraction, so for water the mass percent hydrogen is 11.11%
Hope this helps :)
according to collision theory, what three things are needed in order for a chemical reaction to occur?
Answers
Answer:
the particles must 1. collide
2. with sufficient energy
3. in proper orientation.
Explanation:
hope this helps
What happens to light waves as they enter the Earth's atmosphere?
Answers
Q7. Briefly explain the following:
7.1) Organ donation is considered a noble deed and assists many healthcare clients in reaching an optimal outcome. How is a person able to make an organ donation? As health care professionals, are we allowed to harvest organs for therapeutic reasons from a deceased client who had not consented to organ donation?
7.2) What laws regulate the provision of palliative care?
Feedback
7.3) What is Voluntary assisted dying (VAD)?
Feedback
7.4) Autopsy
Feedback
7.5) What is an Advance Health Directive, and how does it work?
Feedback
7.6) Assessment and management of delirium
Answers
7.1) Organ donation requires consent from the donor. 7.2) Palliative care is governed by laws. 7.3) Voluntary assisted dying (VAD) allows eligible terminally ill individuals to request medical assistance to end their lives. 7.4) Autopsy is a medical examination of a deceased person's body. 7.5) An Advance Health Directive is a legal document specifying healthcare preferences in case of future incapacity. 7.6) Delirium assessment and management involve identifying underlying causes and providing comprehensive care to address symptoms.
7.1) Organ donation is a noble deed that saves many lives. To become an organ donor, a person must indicate their desire to donate their organs on their driver's license, sign an organ donor card, or register online through an organ donor registry website. Healthcare professionals are not allowed to harvest organs for therapeutic purposes from a deceased patient who had not given consent to organ donation.
7.2) Palliative care is regulated by the laws of each country. In general, palliative care is provided in accordance with medical ethics and the principle of beneficence, which is the act of promoting the patient's well-being. Laws and regulations also cover the use of controlled substances and the protection of patients' rights, including the right to refuse treatment.
7.3) Voluntary assisted dying (VAD) is a legal process that allows people who are suffering from a terminal illness to obtain medical assistance to end their lives. The process is voluntary and the person must meet certain eligibility criteria to access the service. The laws governing VAD vary by country and are subject to rigorous legal and ethical scrutiny.
7.4) An autopsy is a medical examination of a deceased person's body to determine the cause of death. Autopsies can be performed with or without the consent of the deceased person's family, depending on the laws of the country or state. Autopsies are an important tool for medical research and can help to improve healthcare outcomes.
7.5) An Advance Health Directive is a legal document that specifies a person's wishes regarding their healthcare if they become unable to make decisions for themselves. The document is prepared in advance and outlines the person's preferences for medical treatment, including whether they wish to be resuscitated or not. The document is legally binding and is used to guide medical decision-making when the person is unable to express their wishes.
7.6) Delirium is a state of confusion and disorientation that can occur in patients with a range of medical conditions. It is important to assess and manage delirium because it can be a sign of an underlying medical problem or a side effect of medication. The management of delirium includes identifying and treating the underlying cause, managing symptoms with medications if necessary, and ensuring the patient's safety.
To know more about Organ donation, refer to the link below:
https://brainly.com/question/33708814#
#SPJ11
What are indicators? How methyl orange and phenolphthalein changes their colour in acidic and basic solutions? How litmus paper changes its colour in different solutions?
Answers
Answer:
See the answer below
Explanation:
In chemistry, indicators are substances that are capable of changing colors with respect to the pH. Each indicator has its characteristic color in acidic pH and another characteristic color in alkaline pH.
Methyl orange indicator appears red in acidic solution and yellow in basic solutions. Phenolphthalein is usually colorless in acidic solutions and appears pink in basic solutions. A red litmus paper will turn blue in alkaline solutions while a blue litmus paper will turn red in acidic solutions.
can someone help me please
Answers
Which group on the periodic table is an atom with 5 valence electrons?Group 4 AGroup 2 AGroup 5 AGroup 15 A
Answers
Explanation:
The atoms on the periodic table are grouped according to having similar properties. Therefore, atoms of group 15 has 5 valence electrons.
Answer:
The last option is correct.
What is the best order to separate this mixture? (The choices below indicate the separation technique and what is separated)
picking - styrofam
magnetism - iron filings
evaporation - salt, water
filter - solids from liquid

Answers
To separate the mixture of water, salt, iron filings, sand, and Styrofoam, you can follow the following steps:
Use a magnet to separate the iron filings. Since iron is magnetic, the magnet will attract the iron filings, allowing you to separate them from the rest of the mixture.Pour the remaining mixture (water, salt, sand, and Styrofoam) into a container. The sand will settle at the bottom due to its higher density.Use filtration to separate the sand from the liquid. Set up a filtration system using filter paper or a sieve. Pour the mixture through the filter, which will allow the liquid (water and salt) to pass through while retaining the sand on the filter.Now you have a mixture of water and salt. You can use evaporation to separate the water from the salt. Pour the liquid into a shallow container and leave it in a well-ventilated area. As the water evaporates, the salt will remain behind.Finally, you are left with the Styrofoam, which can be separated by picking it out manually from the mixture.By following these steps, you can separate the different components of the mixture effectively.
What are the parts of the cell theory?Mark all that apply
Answers
Answer:
Cell theory states that cell living organisms are composed of at least one cell, which are the basic unit of life. Cells are always produced from other living cells, and cannot spontaneously generate
Mineral is --- 1) both neutral and artificial 2)only pure element 3)natural material 4) artificial material
Answers
Answer:Mineral is a natural material.
Explanation:Mineral is a solid natural inorganic substance.
A protozoan gains shelter as it lives in the intestine of a termites, helping the termite to be able to digest the wood that it eats. This symbiotic relationship between the termite and protozoan is known as:
Answers
Answer:
The ecological relationship between termites and the wood-digesting protozoans that live in their gut is an example of mutualism. Thank you for posting your question here at brainly. I hope the answer will help you.
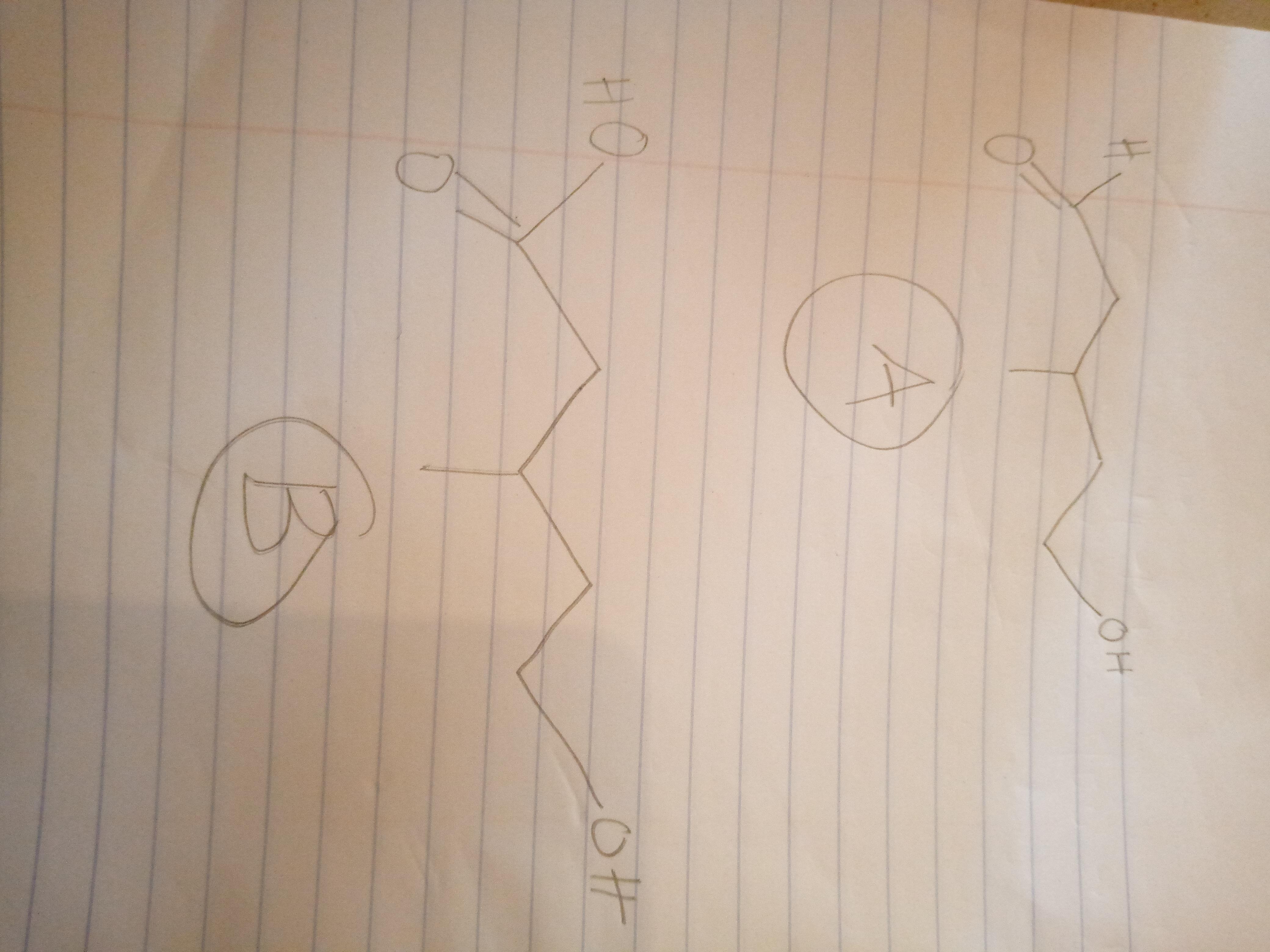
Compound A, C6H12O2, was found to be optically active, and it was slowly oxidized to an optically active carboxylic acid B, C6H12O3, by Ag(NH3)2. Oxidation of A by anhydrous CrO3 gave an optically inactive compound D that reacted with Zn amalgam/HCl to give 3-methylpentane. With aqueous H2CrO4, compound A was oxidized to an optically inactive dicarboxylic acid C, C6H10O4. Give structures for compounds A, B, and C; do not specify stereochemistry.
Answers
Answer:
kindly check the attach file for the drawing of the chemical structures.
Explanation:
So, we are going to start from the compound D, which is stated in the question to be optically active. Therefore, we will have that:
STEP ONE: THE OXIDATION OF COMPOUND A, C6H12O2 TO GIVE COMPOUND C.
The oxidation of compound A,C6H12O2 gives another chemical compound that is chemical compound C which is a optical inactive di-carboxylic acid. The chemical equation is given below:
C6H12O2 + H2Cr2O4 --------------------------------------------> HOOCCH2CHCH3CH2COOH.
STEP TWO: THE OXIDATION OF COMPOUND A, C6H12O2 TO GIVE COMPOUND B.
The oxidation of compound A,C6H12O2 gives another chemical compound that is chemical compound C which is a optically active acid. The chemical equation is given below:
C6H12O2 + Ag(NH3)2^+ -----------------------------> C6H12O3.
Since the question asked us to give the structures of Compound A,B and C there is no need to to show the chemical reaction for compound D.
Kindly check the picture below for the chemical structures.

A substance keeps the same volume, but changes it’s shape according to the container it’s in. Is it a solid, a liquid or a gas?
Answers
Answer:
The substance is in a liquid phase.
Explanation:
The shape of a solid doesn't rely on the container it's in (think of shoes in a shoe box, the shoes maintain their shape).
The shape of a liquid can be changed depending on its container (think of water in a bowl vs. water in a bottle). Regardless of its shape, it maintains the same volume; that is, the amount of water doesn't change.
Gases don't have shapes, so that cannot be the answer either.
The amino acid alanine, CH3CH(NH2)CO2H, has a chiral centre. Illustrate both forms of this isomer and identify the type of isomerism shown.
Answers
Two form of isomer shown by Alanine amino acid is
a) L- alanine
b) D- alanine
Type of isomerism shown is Stereoisomers.
Amino acids are molecules that combine to form proteins. Amino acids and proteins are the constructing blocks of life. When proteins are digested or broken down, amino acids are left. The human body uses amino acids to make proteins to assist the body.
Amino acids are required for the synthesis of body protein and other important nitrogen-containing compounds, which include creatine, peptide hormones, and some neurotransmitters. Despite the fact that allowances are expressed as protein, a the organic requirement is for amino acids.
Learn more about Amino acids here:-https://brainly.com/question/2526971
#SPJ9
How many electrons are in the outermost principal energy level (shell) of an atom of carbon in the ground state?
Answers
Answer:
4 electrons
Explanation:
the valence shell of carbon (for which n=2 ) thus contains 4 electrons.