write your own example of how the carbon cycle works.
Answers
Answer:
Under the picture
Explanation:

Answer:
Carbon moves from fossil fuels to the atmosphere when fuels are burned
Related Questions
A single bond contains_____
Shared electrons.
A.1
B.2
Answers
Answer:
B. 2
Explanation:
A single bond contains 2 shared electrons.
Given example is that of ethane

Answer:
The answer is B. 2
Does anyone know how to determine if a reaction is a redox or not?
2Ca+O2→2CaO2 is the reaction. I've already made it a balanced equation, I just need to determine if its a redox or not.
Thank you!
Answers
The reaction is included in a redox reaction
Further explanation:
Given
Reaction
2 Ca + O₂ → 2 CaO
Required
a redox reaction
Solution
Redox reactions are reactions where there is a change in oxidation number
Oxidation is an increase/increase in oxidation number, while reduction is a decrease/decrease in oxidation number.
Reducing agents are substances that experience oxidation and oxidizing agent are substances that experience reduction
in the above reaction is included in a redox reaction because there is a change in oxidation number
Ca⇒Ca²⁺+ 2e⁻(for balanced equation : 2Ca⇒2Ca²⁺+4e⁻)
Oxidation ( 0 to +2)
O₂+2e⁻⇒O²⁻(for balanced equation : O₂+4e⁻⇒2O²⁻)
Reduction (0 to -2)
NaCl is a solid at room temperature whereas CCI is a liquid. In terms of the bonding types present, explain this difference.
O NaCl is held together by covalent bonds and CCI, is held together by ionic bonds.
The melting point of covalent bonds is lower
than ionic bonds.
ONaCl is held together by covalent bonds and CCI, is held together by ionic bonds.
The melting point of ionic bonds is lower than
covalent bonds.
O NaCl is held together by ionic bonds and CCI, is held together by covalent bonds.
The melting point of covalent bonds is lower
than ionic bonds.
O NaCl is held together by ionic bonds and CCI, is held together by covalent bonds. The melting point of ionic bonds is lower than
covalent bonds.
Answers
Answer:
NaCl is a solid at room temperature whereas CCI is a liquid. In terms of the bonding types present, explain this difference.
O NaCl is held together by covalent bonds and CCI, is held together by ionic bonds.
The melting point of covalent bonds is lower
than ionic bonds.
ONaCl is held together by covalent bonds and CCI, is held together by ionic bonds.
The melting point of ionic bonds is lower than
covalent bonds.
O NaCl is held together by ionic bonds and CCI, is held together by covalent bonds.
The melting point of covalent bonds is lower
than ionic bonds.
O NaCl is held together by ionic bonds and CCI, is held together by covalent bonds. The melting point of ionic bonds is lower than
covalent bonds.
Answer:
NaCl is a solid at room temperature whereas CCI is a liquid. In terms of the bonding types present, explain this difference.
O NaCl is held together by covalent bonds and CCI, is held together by ionic bonds.
The melting point of covalent bonds is lower
than ionic bonds.
ONaCl is held together by covalent bonds and CCI, is held together by ionic bonds.
The melting point of ionic bonds is lower than
covalent bonds.
O NaCl is held together by ionic bonds and CCI, is held together by covalent bonds.
The melting point of covalent bonds is lower
than ionic bonds.
O NaCl is held together by ionic bonds and CCI, is held together by covalent bonds. The melting point of ionic bonds is lower than
covalent bonds.
Answer:
t bonds.
O NaCl is held together by ionic bonds and CCI, is held together by covalent bonds.
The melting point of covalent bonds is lower
than ionic bonds.
O NaCl is held together by ionic bonds and CCI, is held together by covalent bonds. The melting point of ionic bonds is lower than
covalent bonds.Explanation:
Explanation:
Explanation:
solution A is 0.010 M glucose, and solution B is a 0.050 M glucose. The glucose will dialyze to the ?
Answers
Glucose will dialyze from a 0.050 M solution to a 0.010 M solution, glucose molecules will diffuse through the semipermeable membrane, from the higher concentration.
Dialysis is a process in which solute molecules selectively pass through a semipermeable membrane from a region of higher concentration to a region of lower concentration. In this scenario, glucose is the solute and solutions A and B represent different concentrations of glucose.
Since the concentration of glucose in solution B (0.050 M) is higher than in solution A (0.010 M), glucose molecules will dialyze from solution B to solution A. This movement occurs because dialysis allows for the equalization of concentrations, with solute molecules moving from regions of higher concentration to regions of lower concentration.
As a result, solution B to the lower concentration solution A, until the concentration becomes equalized or reaches equilibrium. Therefore, the glucose will dialyze from the 0.050 M solution to the 0.010 M solution.
Learn more about Dialysis here
https://brainly.com/question/30192689
#SPJ11
What name is given to the process by which radioactive atoms break down and change into a different type of atom?
Answers
Explanation: This process is called radioactive decay. Radioactive decay is the process by which an unstable atomic nucleus loses energy by radiation
The equilibrium concentrations for the reaction between SO2 and O2 to form SO3 at a certain temperature are given in the table below. Determine the equilibrium constant and whether the reaction favors reactants, products, or neither at this temperature.

Answers
Answer:
Option B. K = 1.3×10⁴, product favored
Explanation:
Data obtained from the question include:
O2(g) + 2SO2(g) <==> 2SO3(g)
Concentration of O2, [O2] = 0.024 M
Concentration of SO2, [SO2] = 0.015 M
Concentration of SO3, [SO3] = 0.26 M
Equilibrium constant, K =..?
The equilibrium constant, K is simply defined as the ratio of the concentration of products raised to their coefficient to the concentration of the reactants raised to their coefficient.
The equilibrium constant for the above reaction can be written as
K = [SO3]² / [O2] [SO2]²
Inputing the values of [SO3], [O2] and [SO2] the equilibrium constant, K is:
K = [SO3]² / [O2] [SO2]²
K = 0.26² / 0.024 × 0.015²
K = 1.3×10⁴
Therefore, the equilibrium constant K is 1.3×10⁴.
Since the value of the equilibrium constant, K is large and positive, therefore, the reaction favours the product.
How many energy levels does iron have
Answers
Answer:
Number of Energy Levels: 4
First Energy Level: 2
Second Energy Level: 8
Third Energy Level: 14
Fourth Energy Level: 2
Explanation:
Hope It Helps!
Answer:
4 energy levels
Explanation:
1st energy level = 2 electrons
2nd energy level = 8 electrons
3rd energy level = 8 electrons
4th energy level = 8 electrons
find the fine-structure energy levels. what are the degeneracies, and what is the average energy of all states
Answers
The fine-structure energy levels of an atom are determined by the angular momentum of the electrons, and the degeneracies are the number of states with the same energy level.
The average energy of all states can be calculated by summing the degeneracies of each energy level and multiplying by the energy of each level.
For example, if an atom has three energy levels with degeneracies of 2, 4, and 6 and energies of 0.1, 0.2, and 0.3, respectively, the average energy of all states can be calculated as (2 * 0.1 + 4 * 0.2 + 6 * 0.3)/(2 + 4 + 6) = 0.2.Learn more about energy levels at: https://brainly.com/question/14287666
#SPJ4
PLEASEEEE HELPPPPP!!

Answers
Answer:
Its the first one
Explanation:
4 mol is the highest of the Bunch and 4.0L is the lowest making the first answer the correct one
The rate of reaction between two gases increases when the temperature is increased and a catalyst is added. Which statements correctly describe the effect on the reaction?
I. Increasing temperature increases collision frequency
II. Increasing temperature decreases activation energy
III. Adding a catalyst increases the activation energy requirement
IV. Adding a catalyst decreases the activation energy requirement
Group of answer choices
I and III
II and IV
I and IV
II and III
Answers
Answer:
The answer is I and III :)
Have an amazing day!!
Please rate and mark brainliest!!
What is the mass of sulfur in
2.0 moles of Al2(SO4)3?
A. 192.42 g
B. 32.07 g
C. 64.14 g
D. 160.35 g
Answers
Answer:
I guess your answer would be A even thought it is not actually correct it is the closest to being correct.
Explanation:
The molar mass of Al2(SO4)3 is 342.15 g/mol. This means that 1 mole of Al2(SO4)3 has a mass of 342.15 g.To find the mass of sulfur in 2.0 moles of Al2(SO4)3, we first need to determine the number of moles of sulfur in 1 mole of Al2(SO4)3. There are 3 moles of sulfur in 1 mole of Al2(SO4)3, so there are 6 moles of sulfur in 2 moles of Al2(SO4)3.To find the mass of sulfur in 2.0 moles of Al2(SO4)3, we can use the following calculation:Mass of sulfur = (moles of sulfur) x (molar mass of sulfur)
Mass of sulfur = 6 mol x 32.06 g/mol
Mass of sulfur = 192.36 gTherefore, the mass of sulfur in 2.0 moles of Al2(SO4)3 is 192.36 grams.
if 46 g na and 32 g o2 are provided, find the maximum number of moles of sodium oxide produced
Answers
The maximum number of moles of sodium oxide produced is 2.00 moles
The balanced chemical equation for the reaction between sodium and oxygen to form sodium oxide is:
4Na + O2 → 2Na2O
From the equation, we can see that 4 moles of sodium react with 1 mole of oxygen to produce 2 moles of sodium oxide.
To find the maximum number of moles of sodium oxide produced, we need to determine which reactant is limiting.
The molar mass of Na is 22.99 g/mol and the molar mass of O2 is 32.00 g/mol.
Using the given masses, we can calculate the number of moles of each reactant:
moles of Na = 46 g Na / 22.99 g/mol = 2.00 mol Na
moles of O2 = 32 g O2 / 32.00 g/mol = 1.00 mol O2
According to the balanced equation, 4 moles of Na react with 1 mole of O2. Therefore, since we have only 1.00 mol of O2, it is the limiting reactant.
From the equation, we see that 1 mole of O2 reacts to produce 2 moles of Na2O. Therefore, the maximum number of moles of Na2O that can be produced is:
moles of Na2O = (1.00 mol O2) × (2 mol Na2O/1 mol O2) = 2.00 mol Na2O
Therefore, the maximum number of moles of sodium oxide produced is 2.00 moles
Learn more about sodium oxide here:
https://brainly.com/question/29093629
#SPJ11
This graph represents an endothermic reaction. What does it show about the potential energy of reactants and products? Abuzua nunuad in Reaction progress A. The comparison of potential energy depends on what the reactants and products are, B. The potential energy of the products is greater than the potential energy of the reactants C. The potential energy of the reactants equals the potential energy of the products D. The potential energy of the reactants is greater than the potential energy of the products,

Answers
Answer:
The potential energy of the products is greater than the potential energy of the reactants
Explanation:
i just took the test
In an endothermic reaction, activation potential energy of reactants is less than that of the products. Reactants in endothermic reaction are absorbing enough heat energy to overcome this barrier potential. Hence, option B is correct.
What is an endothermic reaction?An endothermic reaction is the one in which heat energy is absorbed by the reactants from the surroundings. In endothermic reactions, the enthalpy change is positive.
The minimum energy that the reactants have to acquire for effective collision and reaction is called the activation energy. By absorbing heat energy reactants becomes more energetic and overcome this activation potential.
Therefore, in an endothermic reaction, the activation potential of products will be higher than that of the reactants and the energy diagram clearly exhibit this transition in energy. Hence, option B is correct.
Find more on endothermic reaction:
https://brainly.com/question/23184814
#SPJ2
What’s the ph of the following equation

Answers
Answer: The pH of [H30+] = 2.4 * 10 -3 M is 2.6
Explanation:
pH = 3 - log 2.4 = 2.6
Which of the following most likely happens when the volume of a gas increases?
-The number of collisions of gas particles remains same.
-The number of collisions of gas particles increases.
-The pressure of the gas remains same.
-The pressure of the gas decreases.
Answers
the pressure of the gas increases
Answer:
The number of collisions of gas particles increases.
Explanation:
Just took the test!!!
Instructions: Use the periodic table to answer the questions below:
1) How many periods are there on the periodic table?
2) How many groups are there on the periodic table?
3) Which element is found in Group 2 and Period 3?
4) Which element is found in Group 17 and Period 2?
5) Which element is found in Group 10 and Period 4?
6) Which element is found in Group 18 and Period 6?
7) Which element is found in Group 1 and Period 7?
8) Which element is found in Group 14 and Period 6?
Answers
Answer:
1. 7 periods
2. 18 groups
3.Magnessium
4.Fluorine
5.Nickel
6.Radon
7.Francium
8.Lead
Explanation:
SURELY SOMEONE HELP it’s urgent plllss I’ll brainlist u/5 star!!! answer the ones u know. :)
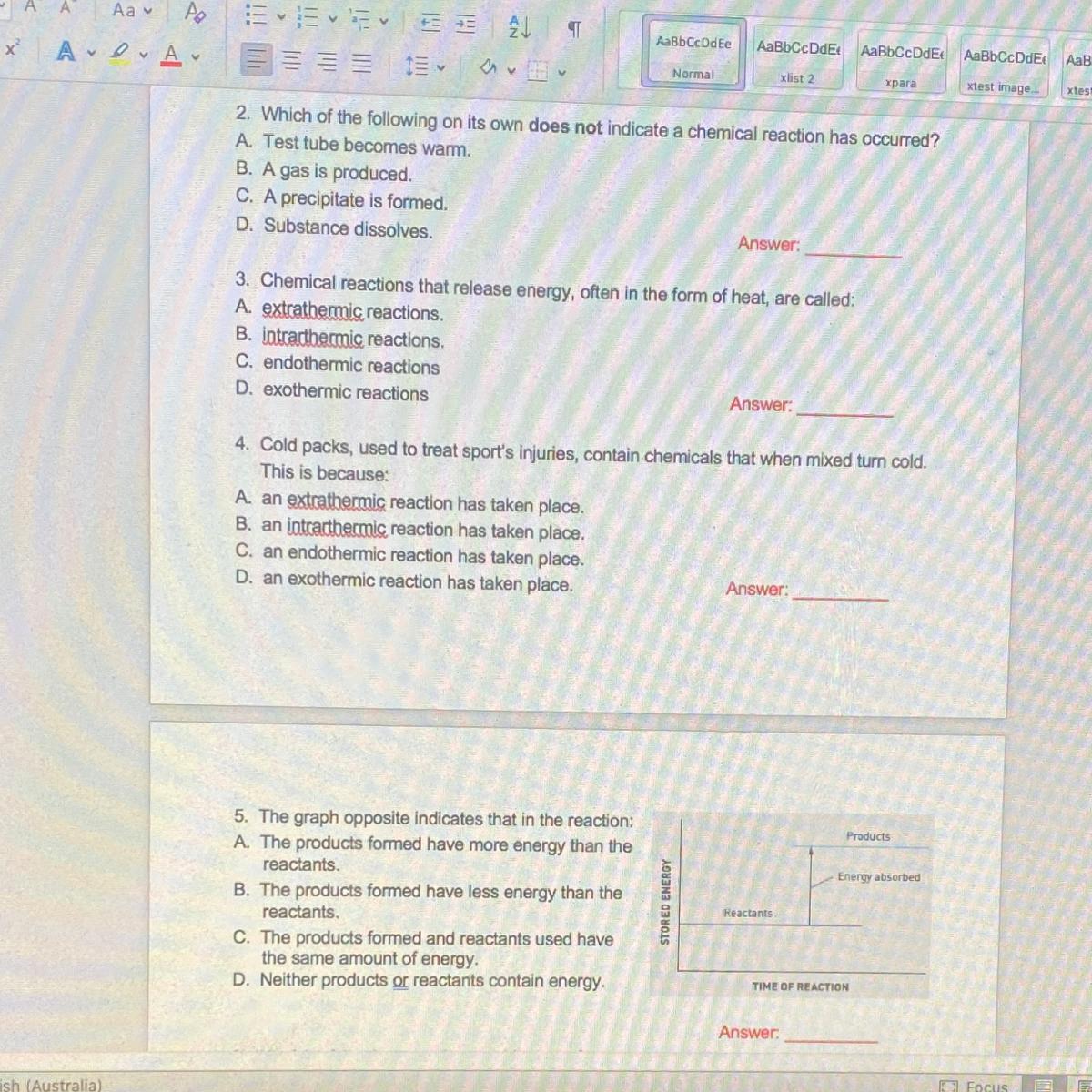
Answers
Answer:
3 exothermic reaction. only that much
Hi,
These are the answers.
• Question 2. C , Substance dissolves
• Question 3. D , Exothermic reaction
• Question 4. C , Endothermic reaction takes place
• Question 5. A , the products formed has more energy than reactants
Hope it helps you... pls mark brainliest if it helped you
Please help me answer these questions. PLEASE


Answers
The moles is 10 moles of diphosphorus hexaoxide
0.75 moles of PbO is produced
The mass of oxygen is 3.7 g
What is the stoichiometry?
If 3 moles of oxygen produces 2 moles of diphosphorus hexaoxide
15 moles of oxygen produces 15 * 2/3
= 10 moles of diphosphorus hexaoxide
2) If 1 mole of oxygen produces 1 mole of PbO
0.75 moles of oxygen will produce 0.75 moles of PbO
3) Number of moles of potassium trioxochlorate V = 9.5 g/122.5 g/mol
= 0.078 moles
If 2 moles of potassium trioxochlorate V produces 3 moles of oxygen
0.078 moles of potassium trioxochlorate V produces 0.078 * 3/2
= 0.117 moles
Mass of oxygen = 0.117 moles * 32 g/mol
= 3.7 g
Learn more about stoichiometry:https://brainly.com/question/29775083
#SPJ1
what type of energy is released when electric charge moves between the clouds and ground
Answers
. The kidneys are _______________ to the liver.
a. Dorsal
b. Ventral
c. Medial
d. Superior
Answers
write a balanced chemical equation for the standard formation reaction of solid water .
Answers
The balanced chemical equation for the standard formation reaction of solid water is: H₂ (g) + 1/2O(g) → H₂O(s)
The standard formation reaction of solid water is: H₂ (g) + 1/2O₂(g) --> H₂O(s)
The balanced chemical equation for the standard formation reaction of solid water is: H₂ (g) + 1/2O(g) → H₂O(s)
The standard enthalpy change of the above reaction can be determined from the enthalpies of formation of the products and reactants using Hess's Law.
Standard enthalpy of formation (∆Hfo) is defined as the amount of heat absorbed or released when one mole of a substance is formed from its constituent elements in their standard states under standard conditions (∆Hfo=0 at 298 K and 1 atm).
Since H₂O(s) is the standard state of water, its standard enthalpy of formation (∆Hfo) is -285.8 kJ/mol. This means that the formation of one mole of solid water releases 285.8 kJ of heat energy.
Learn more about the Hess's Law from the given link-
https://brainly.com/question/16795968
#SPJ11
Which is the balanced version of the half-reaction below that occurs in basic
solution?
CIO CI
A. CIO+ e → Cl
B. CIO + H₂O + 2e → Cl + 20H*
C. CIO + H₂O → CI + 2OH-
D. 2C1O2Cl¯ + 0₂
Answers
The half reactions are the reactions where the reduction and oxidation are shown at the same time.
These are also known as redox reactions.
Redox reactions, as the name tells, is the combination of two types of reaction, the reduction and the oxidation.
The reduction half reaction will show the reduction of either molecules, atoms, oxidation number or charges and the oxidation reaction will show the gain of the same.
In this equation where ClO is reacting to form Cl, is a part of reduction reaction.
This is because the Oxygen is getting detached from the chlorine and the deoxidation of chlorine can be seen.
The de-oxidation of chlorine will result in reduction of the molecule.
Thus the correct answer is option C, ClO + H₂O -> Cl + 2OH⁻ is the half reaction for the given equation.
To learn more about half reaction, refer: https://brainly.com/question/12708131
#SPJ1
Which argument is supported by the information? A. This gene therapy method should be used to improve other senses such as hearing. B. This gene therapy method should be used to prevent blindness that is caused by sun damage. C. This gene therapy method can help improve vision in some patients with the defective gene. D. This gene therapy method can help improve the eyesight of people without an inherited disease
Answers
Based on the information provided, the argument that is supported is:
C. This gene therapy method can help improve vision in some patients with the defective gene.
Gene therapy is a medical approach aimed at treating or preventing genetic disorders by modifying the genetic material of an individual's cells. It involves introducing functional genes or altering existing genes within the cells of a patient to correct or compensate for a genetic mutation or abnormality.
The given information implies that the gene therapy method discussed is effective in addressing a defective gene that impacts vision. Therefore, it suggests that the gene therapy method has the potential to improve vision in individuals with the specific genetic condition.
Hence, C. This gene therapy method can help improve vision in some patients with the defective gene is correct.
Learn more about gene therapy from the link given below.
https://brainly.com/question/13022890
#SPJ4
Calculate the acid ionization constant (Ka) for the acid. Express your answer using two significant figures. IVO AO ? K. = Submit Request Answer A 0.120 M solution of a weak acid (HA) has a pH of 3.28. You may want to reference (Pages 737 - 745) section 16.6 while completing this problem.
Answers
Answer : The acid ionization constant (Ka) for the given acid HA is 1.1 x 10^(-5), rounded to two significant figures.
To calculate the acid ionization constant (Ka) for the given acid HA, we must first find its pH using the given concentration of the solution. Then, we can use the pH to find the concentration of H+ ions in the solution. Finally, we can plug these values into the expression for Ka to solve for the acid ionization constant.
The pH of the 0.120 M solution of HA is given to be 3.28. This means that [H+] = 10^(-pH) = 10^(-3.28) = 5.01 x 10^(-4) M.
Now, we can use the expression for Ka: Ka = [H+][A-]/[HA], Since HA is a weak acid, we can assume that it dissociates as follows: HA + H2O ⇌ H3O+ + A- This means that [A-] = [H3O+], and [HA] = initial concentration of the acid (0.120 M) - [H3O+].
Substituting these values, we get: Ka = (5.01 x 10^(-4) M)^2 / (0.120 M - 5.01 x 10^(-4) M) = 1.1 x 10^(-5). Therefore, the acid ionization constant (Ka) for the given acid HA is 1.1 x 10^(-5), rounded to two significant figures.
Know more about acid ionization constant here:
https://brainly.com/question/4110687
#SPJ11
explain why you would expect the molar heat capacity at constant pressure of an ideal gas to be larger than the molar heat capacity at constant volume.
Answers
The thermal capacity under a certain pressure Since the substance expands and produces energy when heat is applied at a constant pressure, CP is greater than the heat capacity at constant volume CV.
Why does the first law of thermodynamics demonstrate that CP Cv R and that specific heat at constant pressure is greater than that at constant volume?In the first instance, the temperature of the gas must be raised with greater heat. Since it requires more energy to raise the temperature by one unit in the former situation, CP, or specific heat at constant pressure, is greater than CV, or specific heat at constant volume.
To know more about heat capacity visit:-
https://brainly.com/question/28302909
#SPJ4
Au+HCL(aq) Complete the chemical Equation
Answers
The complete balanced chemical equation is:
2\(Au\) + 6\(HCl\) (aq) → 2\(Au\)\(Cl_{3}\) (aq) + 3\(H_{2}\) (g)
What is a chemical equation?Chemical equations provide the reactants, products, and mole ratios of each component involved in the reaction and are a symbolic depiction of a chemical reaction. The reactants are written on the left side of the arrow, while the products are written on the right side, using chemical symbols and formulas. The coefficients in front of the formulas show the number of molecules involved and the arrow shows the reaction's direction. Chemical equations serve to explain the underlying chemical processes and forecast the volume of products that will be created in a reaction. They are a crucial tool in the study of chemistry and are essential to chemical reactions and their uses in a variety of sectors.
To know more about chemical equations, check out:
https://brainly.com/question/5880605
#SPJ1
what happens if the spots are made too large when preparing a tlc plate for development?
Answers
If the spots on a TLC plate are made too large when preparing it for development, there are a few potential consequences.
First, the resolution of the separation may be compromised, as the larger spots will not allow for as much separation between different components in the sample. Second, the spots may merge together as they migrate up the plate during development, making it difficult or impossible to distinguish between different components.
Finally, the larger spots may saturate the stationary phase on the TLC plate, which can lead to poor retention and separation. It is important to carefully control the size of spots when preparing a TLC plate to ensure accurate and effective separation of components in the sample.
To know more about TLC plate refer here:
https://brainly.com/question/13086570
#SPJ11
A gaseous mixture of methane , ethane and propane has partial pressures respectively of 95 kPa,105 kPa and 50 kPa. What is the mass percentage of methane in the mixture?
Answers
The mass percentage of methane in the gaseous mixture of methane, ethane, and propane is approximately 43.5%.
The mass percentage of a gas in a mixture can be calculated by dividing the mass of the gas by the total mass of the mixture and then multiplying by 100%.
To calculate the mass of each gas, the ideal gas law, PV = nRT, can be used. Here, P is the partial pressure of the gas, V is the volume of the mixture, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature.
Assuming a constant temperature and volume, the number of moles of each gas can be determined from the partial pressures of the gases:
n_methane = (95 kPa * V) / (R * T)
n_ethane = (105 kPa * V) / (R * T)
n_propane = (50 kPa * V) / (R * T)
The mass of each gas can be calculated using the molar mass of the gas:
m_methane = n_methane * M_methane
m_ethane = n_ethane * M_ethane
m_propane = n_propane * M_propane
Where M is the molar mass of the gas. The molar mass of methane is 16.04 g/mol, the molar mass of ethane is 30.07 g/mol, and the molar mass of propane is 44.10 g/mol.
The total mass of the mixture can be calculated as the sum of the masses of each gas:
total mass = m_methane + m_ethane + m_propane
Finally, the mass percentage of methane in the mixture can be calculated as:
mass percentage of methane = (m_methane / total mass) * 100%
Approximating the values, the mass percentage of methane in the gaseous mixture of methane, ethane, and propane is approximately 43.5%.
To learn more about propane, visit:
https://brainly.com/question/25008296#
#SPJ11
i. If a solution of formic acid contains 0.2 MHCHO2, 0.006 M CHO₂, and 0.006 MH*, what is the
Ka of the acid?
Answers
Ka of formic acid is 1.8·10⁻⁴ M.
Briefing :Chemical dissociation of formic acid in the water:
HCOOH(aq) ⇄ HCOO⁻(aq) + H⁺(aq).
K = [HCOO⁻] · [H⁺] / [HCOOH].
equilibrium concentration of [HCOOH] = 0.2 M,
equilibrium concentration of [H⁺] = 0.0.6 M,
equilibrium concentration of [HCOO⁻] = 0.006 M
K = (0.006 M)² / 0.2 M.
K = 0.00018 M.
K = 1.8·10⁻⁴ M.
What happens in a dissociation reaction?When one molecule splits into two smaller ones, a dissociation reaction takes place, which results in a loss of energy. Decomposition reactions, which are also known as dissociation reactions, are those in which a large molecule is broken down into smaller products.
To know more about energy :
https://brainly.com/question/1932868
#SPJ9
In which of the following will the density increase?
Group of answer choices
An iron bar is heated.
A lead weight is moved from sea level to the top of a high mountain.
A sample of water is frozen.
A diamond is submerged in water.
A sample of chlorine gas is compressed.
Answers
Good luck
Regards
BLACKSHARK