x is an unknown element which forms an acid, hxo3. The mass of 0. 0133 mol of this acid is 1. 123 g. Find the atomic mass of x and identify the element represented by x. The element x is.
Answers
The atomic mass of the unknown element (x) that forms the acid HXO₃ is 63.55 g/mol and the element represented by x is Copper (Cu).
The formula for the given acid, HXO₃, is suggestive of a tertiary acid containing three oxygen atoms. For a compound with 0.0133 mol, the mass is 1.123 g.1 mol of a compound with mass 1.123 g = 1.123 / 0.0133 = 84.36 g/mol. Therefore, one molecule of HXO₃ weighs 84.36 g/mol.
For this acid, we can establish the following equation: HXO₃ = H + X + 3O → Atomic mass of X = [(Atomic mass of H) + (Atomic mass of O) × 3] - Atomic mass of HXO₃; Atomic mass of H = 1.01 g/mol; Atomic mass of O = 16.00 g/mol; Atomic mass of HXO₃ = 84.36 g/mol; Atomic mass of X = (1.01 + 16.00 × 3) - 84.36 = 63.55 g/mol. Based on this, we can conclude that the element X is Copper (Cu), which has an atomic mass of 63.55 g/mol.
Learn more about atomic mass here:
https://brainly.com/question/29117302
#SPJ11
Related Questions
An unknown mass of silver is heated, and then placed into a calorimeter containing 250.0 grams of water at 6.50 °C. The silver and the water reach thermal equilibrium at 23.35 °C. What is the amount of HEAT lost by the the silver sample? (Note: Specific heat of water is 4.18 J/g·ºC).
Answers
q water = 17625.1
-17625.1 would be the total amount of heat lost by the silver
What happened to the fabric strip when it was treated with the Test Identification Stain? How might this dye be used?
Answers
a. When the fabric strip was treated with the Test Identification Stain, it underwent a chemical reaction that caused a color change.
b. This dye can be used in forensic science to identify different types of fibers or to determine if a particular fabric was present at a crime scene.
When the fabric strip was treated with the Test Identification Stain, it experienced a change in color as the stain reacted with the fibers in the fabric. This color change indicates the presence of specific components or substances within the fabric.The dye in the stain reacted with the fibers of the fabric, resulting in a visible color change.
The dye can be used as a diagnostic tool to identify and distinguish between various types of fibers, materials, or even contaminants in the fabric. By observing the color change and comparing it to known standards, you can gain valuable information about the composition and properties of the fabric.
Learn more about dye: https://brainly.com/question/11565052
#SPJ11
describe how to identify the smell of gas in the laboratory
Answers
Answer:
When you are in the laboratory and take a direct sniff the chemicals you are using, you run the risk of damaging your mucous membranes or your lungs. When its necessary to smell chemicals in the lab, the proper technique is to cup your hand above the container and waft the air towards your face.
Gas is a naturally odourless substance, but the completely harmless artificial smell is added to make it more detectable. The substance is called mercaptan and gives off a strong sulphur like smell.
37.9 grams of an unknown substance undergoes a temperature increase of
25.0*C after absorbing 969 J. What is the specific heat of the substance?
Answers
Answer:
1.023 J / g °C
Explanation:
m = 37.9 grams
ΔT = 25.0*C
H = 969 J
c = ?
The equation relating these equation is;
H = mcΔT
making c subject of formulae;
c = H / mΔT
c = 969 J / (37.9 g * 25.0*C)
Upon solving;
c = 1.023 J / g °C
Baking soda is sodium bicarbonate, NaHCO3, and vinegar is primarily acetic acid, HC2H3O2. When baking soda is added to vinegar, the resulting reaction produces a tremendous amount of gas, as shown in this video. NaHCO3(s) + HC2H3O2(aq) rightarrow Complete this equation for the reaction of NaHCO3(s) with HC2H3O2(aq). Include phase symbols. NaHCO3(s)+ HC2H3O2(aq) rightarrow
Answers
NaHCO3(s) + HC2H3O2(aq) → CO2(g) + H2O(l) + NaC2H3O2(aq)
This equation represents the reaction of baking soda (sodium bicarbonate) with vinegar (acetic acid) to generate carbon dioxide gas, water, and sodium acetate.
The balanced equation for the reaction of NaHCO3(s) with HC2H3O2(aq) including phase symbols is
NaHCO3(s) + HC2H3O2(aq) → CO2(g) + H2O(l) + NaC2H3O2(aq)
Baking soda, also known as sodium bicarbonate, is a white, crystalline powder with the chemical formula NaHCO3. It is an alkaline substance that neutralizes acids.
Vinegar is mostly composed of acetic acid, HC2H3O2, which is a weak acid. Vinegar has a sour flavor and a strong smell due to the presence of acetic acid.
NaHCO3(s) + HC2H3O2(aq) → CO2(g) + H2O(l) + NaC2H3O2(aq) This equation represents the reaction of baking soda (sodium bicarbonate) with vinegar (acetic acid) to generate carbon dioxide gas, water, and sodium acetate. When the baking soda and vinegar are combined, a chemical reaction occurs, causing carbon dioxide gas bubbles to form. This is due to the reaction between the acid and base in the mixture, which generates carbon dioxide gas as a byproduct. This reaction is commonly used in baking as a leavening agent to make cakes, muffins, and other baked goods rise.
Learn more about balanced equation from:
https://brainly.com/question/26694427
#SPJ11
3.20 g of magnesium reacts with hydrochloric acid, generating
hydrogen gas according to the reaction below. The hydrogen
gas is collected over water.
Mg(s) + 2HCl(aq) MgCl2(aq) + H2(8)
If 2.97 L of hydrogen gas is collected at 760.0 mmHg and
20°C, what was the percent yield of the reaction?
Answers
Answer: 93.8
Explanation: hope you have Wonderful grades!!
plz answer (d) explain

Answers
Cu2+ is reduced to Cu.
Change in oxidation state is from +2 to 0


is calcium a metal? yes or no answer please
Answers
A 100.0 mL sample of 0.180 M HClO 4 is titrated with 0.270 M LiOH. Determine the pH of the solution after the addition of 75.0 mL of LiOH.
2.65
1.89
11.35
13.06
12.1
Answers
The pH of the solution after the addition of 75.0 mL of LiOH is calculated as 12.1 .
Option E is correct.
The pH of an answer is a proportion of hydrogen particle focus, which thusly is a proportion of its causticity. Unadulterated water separates somewhat into equivalent groupings of hydrogen and hydroxyl (OH−) particles
100 ml of 0.180 M HClO₄ = \(\frac{0.180mol}{1 L}\) × 0.1 L
= 0.018 mol
75 ml of 0.270 M LiOH = \(\frac{0.270 mol}{1 L}\) × 0.075 L
= 0.02025 mol
HClO₄ + LiOH ⇒ LiClO₄ + H₂O
0.018 0.0202 0 0
- 0.018 -0.018 + 0.018
pOH = - log [ OH ⁻]
= - log ( 0.0126)
= 1.90
[OH⁻] = 0.0022/ 0.175 M
= 0.0126 M
pH = 14 - pOH
= 14 - 1.90
= 12.1
pH is defined in what way?a measure of a substance or solution's acidity or basicity. The pH scale ranges from 0 to 14. A pH of 7 is neutral on this scale, meaning that it is neither acidic nor basic. A pH worth of under 7 methods it is more acidic, and a pH worth of in excess of 7 methods it is more essential.
Why is pH so crucial?The chemical conditions of a solution are reflected in the pH, an important quantity. Chemical behavior, microbial activity, the availability of nutrients, and biological functions are all influenced by pH.
Learn more about pH level:
brainly.com/question/172153
#SPJ4
Gas stored in a tank at 273 K has a pressure of 388 kPa. The safe limit for pressure is 825 kPa. At what temperature will the gas reach this pressure?
Answers
580.47 Kelvin
Gas stored in a tank at 273 K has a pressure of 388 kPa. The safe limit for pressure is 825 kPa.
temperature will the gas reach this pressure =
as per above statement, we have got
p1 = 388 kPa P2 = 825 kPa
T1 = 273 K T2 =
as per ideal gas equation PV = nRT
since n, V , R is constant , so we ignore them,
now equation will become
P1/T1 = P2T2
now putting the values
388/273 = 825/T2
T2 = 825 * 273 ÷ 388
T2 = 224,225/388
T2 = 580.47 Kelvin
To know more about ideal gas equation visit :
https://brainly.com/question/4147359
#SPJ9
Explain the trend as you move across a row of the periodic table for each of the following atomic
properties using your understanding of effective nuclear charge.
a. Atomic radius
b. Ionization energy/electronegativity
Answers
a) atomic radius decreases when moving across a period and increased when going down a group.
b) ionization energy increases when moving from left to right across and element period.
Vocab explanations:
groups: The columns of the periodic table are called groups.
periods: The horizontal rows are called periods.

The bond order of any molecule containing equal numbers of bonding and antibonding electrons is ________.
Answers
The bond order for any molecule having an equal number of bonding and antibonding electrons is zero.
The "degree" of the bond is best described by the bond order. For instance, the bond order of a triple bond is 3, while the bond order of double bond is 2. One can imagine that each electron contributes to the bonding or antibonding characteristic. Half of the bonding feature is contributed by each electron in a bonding molecular orbital. Half of the antibonding feature is contributed by each electron in an antibonding molecular orbital. Bond order is zero when bonding and antibonding character are equal. Therefore, the bond order is 0 when the number of bonding and antibonding electrons is equal.
Mathematical proof is also possible.
Bond Order: ½(Antibonding electrons - bonding electrons)
When both are equal. Suppose number of both electrons is x then,
Bond Order: ½(x – x) = 0
Want to know more about bond orders visit;
https://brainly.com/question/16526043
#SPJ4
Matter changing from a solid to a liquid is called
Answers
SOMEONE HELP ME PLEASE ILL GIVE BRAINLY!
Is the following picture an example of a chemical change or a physical change?
A chemical change, because the atoms are rearranging to form new molecules.
A physical change, because the molecules are staying the same.
A chemical change, because the molecules are staying the same.
A physical change, because the atoms are rearranging to form new molecules.

Answers
Answer:
It would be a physical change, because the atoms are rerranging to form new molecules!
Explanation:
What is the formula for dinitrogen pentoxide?
A) NO5
B) N5)2
C) N2O5
D)N2O7
Answers
Explanation
B) N5)2 hopefully
The pH of the ocean is around 8.1, is the ocean considered a
buffer? Why or Why not?
Answers
Yes, the sea is considered a buffer.
A buffer is a solution that resists pH changes when acids or bases are added. The buffering capacity of the ocean allows it to maintain a relatively stable pH even when acids and bases are added.
The ocean's buffering capacity is primarily due to the presence of dissolved compounds such as bicarbonate (HCO3-) and carbonate (CO32-). These compounds act as both weak acids and bases, accepting and releasing hydrogen ions (H+) to maintain pH balance. When carbon dioxide (CO2) in the atmosphere dissolves in seawater, carbonic acid (H2CO3) is produced and decomposed into bicarbonate ions and hydrogen ions.
This transformation helps prevent a rapid drop in pH as excess hydrogen ions combine with carbonate ions to form bicarbonate ions, which can reduce overall acidity.
When alkali such as hydroxide ions (OH-) is added to the ocean, excess hydroxide ions combine with hydrogen ions to form water molecules, reducing alkalinity.
The presence of these dissolved compounds and their interconversion reactions stabilize the pH of the ocean, making it less susceptible to rapid changes in acidity or alkalinity. This buffering capacity is essential for the survival and maintenance of marine life, as many organisms are sensitive to changes in pH.
To know more about PH refer to this link
https://brainly.com/question/12609985
Competition of mineral formation! Dolomite, CaMg(CO3)2, is another common carbonate rock, with logK=−17.09 and the reaction as follow: CaMg(CO3)2⇌Ca2++Mg2++2CO32− In the water sample of question lb, if [Mg2+]∼0.10mmolL−1, which mineral (calcite or dolomite) would form first? Hint: Calculate the Q/K ratios for each mineral. This ratio is also commonly referred to as the saturation index; the mineral with higher SI will be more likely to precipitate first.
Answers
By performing the necessary calculations and comparing the Q/K ratios, we can determine whether calcite or dolomite would form first in the given water sample with [Mg2+]∼0.10 mmol/L.
To determine which mineral, calcite or dolomite, would form first in the given water sample with [Mg2+]∼0.10 mmol/L, we need to calculate the saturation index (SI) for each mineral by comparing the Q/K ratios. The mineral with the higher SI will be more likely to precipitate first.
The saturation index (SI) is calculated by comparing the ion activity product (Q) with the equilibrium constant (K) for a particular mineral. In this case, we have the equilibrium reaction: CaMg(CO3)2⇌Ca2++Mg2++2CO32−.
For calcite, the Q/K ratio can be calculated using the concentration of Ca2+ and CO32− ions in the water sample. Since dolomite contains both Ca2+ and Mg2+ ions, we need to consider the concentration of Mg2+ as well.
By comparing the Q/K ratios for calcite and dolomite, we can determine which mineral has a higher saturation index (SI). The mineral with the higher SI will be more likely to precipitate first.
Learn more about equilibrium constant here:
https://brainly.com/question/29809185
#SPJ11
What is the claim in a literary analysis?
a reason that makes your opinion believable
an emotional statement of opinion
a reasonable, debatable opinion about the work
a summary of the factual evidence
Answers
An argumentative, plausible view of the literary work being evaluated is the claim in a literary analysis.
The claim in a literary analysis, which is an interpretation of a literary work, is the author's argument or viewpoint regarding the relevance or meaning of the work.
The assertion needs to be clear, debatable and backed up by textual evidence.
Literary analysisThe claim in literary analysis is the main viewpoint or argument that the author is advancing regarding the relevance or meaning of the literary work under consideration.
The assertion should be a reasonable, disputed opinion that can be backed up by textual evidence, and it should be sufficiently detailed to be convincing and understandable to the reader.
For instance, in an interpretation of William Shakespeare's play "Hamlet," a writer can contend that, rather than a lack of courage, Hamlet's hesitation to exact revenge on his father's murderer stems from his desire for justice and his battle with indecision.
This assertion is both plausible and problematic because different readers or critics may interpret Hamlet's actions differently.
learn more about the literary analysis here
https://brainly.com/question/9965425
#SPJ1
Type the correct answer in each box. express your answer to two significant figures. you form water vapor by mixing oxygen and hydrogen at 730°c in a 5.4-liter container. this is the equation for the reaction: o2(g) 2h2(g) → 2h2o(g). the partial pressure of oxygen before the reaction is 122.3 kilopascals, and there is excess hydrogen. how many moles of water are formed? the reaction produces moles of water.
Answers
The number of moles of water vapor formed in the reaction is 0.158 moles
What is the ideal gas equation?The ideal gas equation shows the relationship between number of moles, temperature, volume and pressure.
Now;
P = 122.3 kilopascals or 1.21 atm
V = 5.4 L
T = 730°c or 1003 K
n = ?
R = 0.082 atmLK-1mol-1
PV = nRT
n = PV/RT
n = 1.21 * 5.4/0.082 * 1003
n = 6.53/82.2
n = 0.079 moles
The equation of the reaction is; O2 (g) + 2H2(g) ---->2H2O(g)
If 1 mole of O2 formed 2 moles of H2O
0.079 moles of O2 yield x moles of H2O
x = 0.079 * 2/1
= 0.158 moles
Learn more about ideal gas equation:https://brainly.com/question/4147359
#SPJ1
Ammonia gas and water (H20) reaction to form household ammonia, which contains NH4 and OH ions. What is the formula for ammonia gas? Name the elements in the compound. In what ratio are they presented
Answers
Answer:
NH3
Explanation:
The formula for ammonia gas is NH3. Its appearance is a colorless gas.
Ammonia gas is formed by the balanced chemical reaction between nitrogen and hydrogen elements. The balanced chemical equation for the formation of ammonia gas is as follows:
N2+3H => 2NH3
So, Nitrogen and Hydrogen are present in the ration of 2:6 or 1:3.
Answer:
lol
Explanation: lol
Which of the following is an example of using creativity while recording measurements during an experiment? (5 points) a Writing a hypothesis b Organizing data in tables c Validating results by repetition d Interpreting results based on data
Answers
Answer:A
Explanation:
How much voltage is required to run 1.6 A of current through a 240
resistor? Use AV = IR.
O A. 380 V
O B. 6.7 x 10-3 v
O C. 150 V
O D. 2.6 x 10-3 v
Answers
Answer:
answer is not there
Explanation:
V=1.6×240
V=384volt
which step you should take before performing a scientific investigation
A. print the directions after you complete the experiment
B. test out all of your emergency equipment you may need
C. clarify any confusing information in the instructions
D. Purchase the equipment and chemicals you will need
Answers
Answer:
D. Purchase the equipment and chemicals you will need
Explanation:
a reducing chemical reaction . group of answer choices reduces the compound to a simpler form adds an electron to the substrate removes a hydrogen atom from the substrate is a catabolic reaction
Answers
A reducing chemical reaction adds an electron to the substrate.
Reduction is a term used to describe the yield of electrons, the accumulation of hydrogen, or the reduction of oxygen from the substrate.
When a reactant accumulates electrons during a reaction, it is called reduction. Reduction reactions include the addition of electrons to a substrate and the donor of the electrons is a reducing agent that itself brings oxidization. Therefore a reducing chemical reaction retains the addition of electrons to a substrate.
Oxidation-reduction (redox) reactions consist of the transfer of electrons between compounds while catabolic reactions are the type of reactions in which complex substances are split down into simpler units with the discharge of energy. Therefore these options are incorrect
To learn more about reduction reaction; click here:
https://brainly.com/question/27961005
#SPJ4
ANSWER QUICK PLEASE I GIVE BRANLIEST

Answers
Answer:
Answer 3 is the correct answer.
What are the features that allow you to identify an ionic compound based on its chemical formula
Answers
Answer:
first identify the cation and write down its symbol and charge. Then, identify the anion and write down its symbol and charge.
Explanation:
what is the change in internal energy (in j) of a system that absorbs 0.335 kj of heat from its surroundings and has 0.966 kcal of work done on it? give your answer in scientific notation.
Answers
The change in the internal energy is 4.395 kj
What is internal energy?
Rising temperature and changes in state as well as phase from solid state to liquid as well as liquid to gas cause an increase in internal energy. Planetary bodies could be viewed as a fusion of heat engines and heat reservoirs. Internal energy E is stored in the heat reservoirs, and some of it is converted into different kinds of mechanical, electrical, and chemical energies by the heat engines. The sum of the kinetic energy brought on by the molecule's movement and the potential energy brought on by the vibrational motion as well as electric energy of atoms inside of molecules makes up the internal energy U of the a system or a body with clearly defined boundaries. The energy contained in every chemical bond is also referred to as internal energy.
Given;
Q = +0.335 kj
W = - 0.966 kcal = -966 cal = - 4.04kj
del E int = +0.355 - [-4.04]
= 4.395 > 0
{Increase in internal energy}
To learn more about internal energy from the given link
https://brainly.com/question/25737117
#SPJ4
what is the atomic number for an element whose mass number is 78, which contains 48 neutrons per atom?
Answers
The atomic number of the element is 30.
The atomic number of an element is the number of protons in its nucleus. The atomic number is a term used in chemistry to describe the number of protons in the nucleus of an atom. It is represented by the symbol "Z" and is unique for each element on the periodic table. The atomic number determines the chemical properties of an element and is used to organize elements on the periodic table.
The mass number is the sum of the number of protons and neutrons in the nucleus. Therefore, to find the atomic number, we can subtract the number of neutrons from the mass number: 78 - 48 = 30. So, the atomic number for this element is 30.
Learn more about atomic number at https://brainly.com/question/11353462
#SPJ11
help me pleaseee. ty if you dooo :))
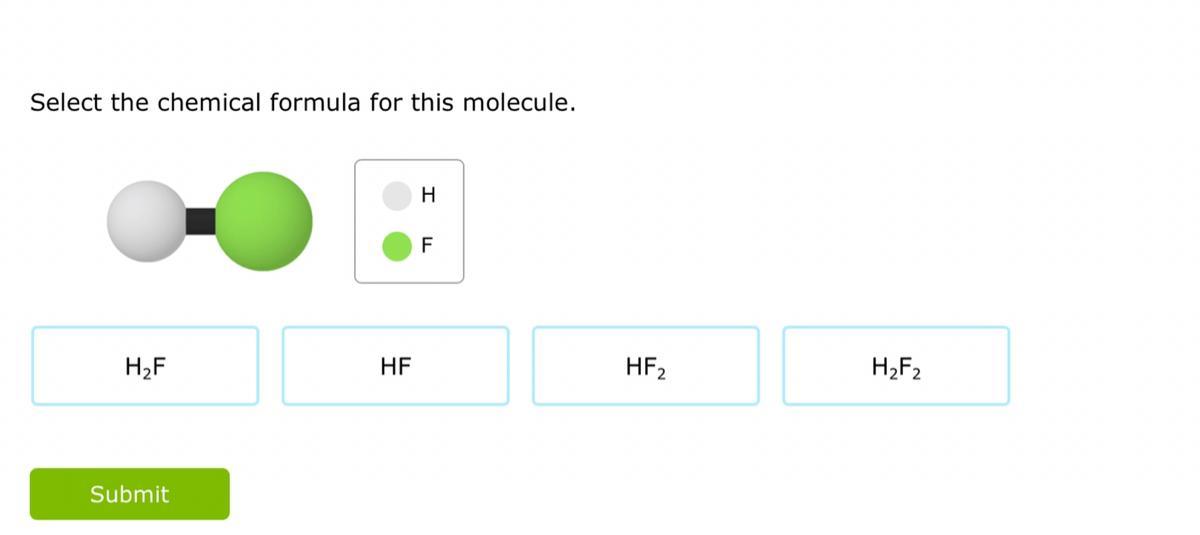
Answers
\(\huge \fcolorbox{black}{red}{♛answer♛}\)
Hydrogen Fluoride / HF
\(\huge\sf\underline{\underline{\red{❥︎ Thanks}}}\)
According to the infrographic, what happens to carbon after trees respire?
A. It cleans the soil of fungi.
B. It is stored in the roots and branches
C. It is released into the atmosphere.
D. It is turned to sugar and minerals.
