your friend has just started a mining company and has asked you to help and talk to his newly recruited staff about the formation, characteristics and identification of rocks and minerals. prepare a presentation (not more than 200 words) to be delivered to the staff about rocks and minerals and how they can identify them.
Answers
The formation, characteristics and identification of rocks and minerals are are naturally occurring substances. presentation can be done as, check the details in the explnation.
What is the formation, characteristics and identification of rocks and minerals presentation?Rocks and minerals are naturally occurring substances that are composed of various elements and compounds. Rocks are made up of two or more minerals, while minerals are made up of one or more elements. Rocks can be identified by their physical characteristics, such as color, texture, and hardness.
. Minerals can be identified by their chemical composition, crystal structure, and physical properties. Common characteristics used to identify rocks and minerals include color, streak, luster, hardness, cleavage, and fracture.
Learn more about rocks at:
https://brainly.com/question/14021434
#SPJ1
Related Questions
Pls help with question 10

Answers
Answer:
Formula/Molecular Mass = 147.77415g/mol = 148g/mol (to 3 significant figures)
Explanation:
Given the Law of the Conservation of Mass, the amount of H2O produced will be equal to 10.00g-4.40g = 5.6g
Given equation n=m/M ,
n(H2O)= 5.6/ (1.008×2)+(15.999) = 0.318520677 moles
∴ x=0.318520677 moles
∴ Molecular mass (hydrated sodium sulfate) =
= (22.990×2)+(32.06)+(15.999×4)+[0.318...((1.008×2)+(15.999))]
= 147.77415g/mol
Answer:
Answer is in the attachment:
x=10
Explanation:

How many parts of sodium chloride 0.45% are in 100 parts of solution?
Select one:
0.045
0.45
4.5
45
Answers
There are 0.45 parts of sodium chloride in 100 parts of the 0.45% solution.
When we talk about a solution, we refer to a homogeneous mixture of two or more substances. The substance that dissolves in the solution is called the solute, while the substance in which the solute dissolves is called the solvent.
In this case, the solute is sodium chloride, which is a common salt, and the solvent is water. Sodium chloride 0.45% refers to the concentration of the salt in the solution. It means that there are 0.45 grams of sodium chloride per 100 milliliters of solution.
When we say "parts," we can refer to any unit of measurement, such as grams or milliliters. Therefore, we can say that there are 0.45 parts of sodium chloride in 100 parts of solution. This means that in a liter of solution (1000 milliliters), there are 4.5 grams of sodium chloride.
In conclusion, the answer to the question is 0.45 parts of sodium chloride in 100 parts of solution. This concentration is commonly used in medical applications, such as intravenous fluids, to replace lost fluids and electrolytes in the body.
learn more about sodium chloride Refer: https://brainly.com/question/19371678
#SPJ11
How many formula units are in 0.25 mole of NaCl?
Answers
Answer:
The meaning and usefulness of the mole Page 2 2 • One mole of NaCl contains 6.022 x 1023 NaCl formula units. Use the mole quantity to count formulas by weighing them. The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams.
Explanation:
Formula Units = mole x Avogadro's Number
Formula Units of NaCl = mole NaCl x Avogadro's Number
Formula Units of NaCl = 0,25 mole x 6,022 x 10²³ molecule
Formula Units of NaCl = 1,5055 x 10²³ molecule
A reaction has an enthalpy change of − 71 kJ mol − 1 and an entropy change of − 58 J K − 1 mol − 1 . At what temperature does this exothermic reaction cease to be spontaneous?
Answers
To determine the temperature at which an exothermic reaction ceases to be spontaneous, we need to calculate the Gibbs free energy change (ΔG) and use the equation ΔG = ΔH - TΔS.
Given that ΔH = -71 kJ/mol and ΔS = -58 J/K·mol, we can calculate ΔG at different temperatures to determine the temperature at which the reaction becomes non-spontaneous.
At a temperature of 0 K, ΔG = ΔH, since TΔS = 0. Thus, ΔG = -71 kJ/mol.
As the temperature increases, TΔS becomes more negative, which means that ΔG becomes more negative, making the reaction more spontaneous.
At a certain temperature, however, ΔG will become positive, which means that the reaction is no longer spontaneous and will not proceed on its own. This temperature can be found by rearranging the equation ΔG = ΔH - TΔS to T = ΔH / ΔS, and substituting the known values for ΔH and ΔS:
T = ΔH / ΔS = -71 kJ/mol / (-58 J/K·mol) = 1230 K
So, the reaction will cease to be spontaneous at a temperature of approximately 1230 K.
What type of mechanical mixture is pop
Answers
Hope this helped!
I need help with this I’m not sure on how to do it

Answers
Stoichiometry is the study and calculation of quantitative (measurable) relationships of the reactants and products in chemical reactions (chemical equations).
QUESTION 18;
4 moles of ammonia reacts to produce 2 moles of nitrogen gas
0.68 moles of ammonia will produce 0.34 moles of nitrogen gas.
Mass of nitrogen gas = 0.34 moles × 34g/mol = 11.56g
QUESTION 19;
1 mole of zinc reacts with 2 moles of hydrochloric acid
34.5 grams of Zn is equivalent to 34.5/65.39 = 0.53 moles
0.53 moles will react with 1.06 moles of HCl.
Learn more about stoichiometry at: https://brainly.com/question/29775083
#SPJ1
a. 1.73 m =
cm
a. 7,651.27 m =
km
Answers
Answer:
A. 1.73 m = 172 cm
B. 7,651.27 m = 7.65127 Km
2
4.6 g of sodium reacts with chlorine to produce 11.7 g of sodium chloride.
What mass of chlorine reacted?
Answers
Mass of chlorine reacted : 7.1 g
Further explanationThe reaction equation is the chemical formula of reagents and product substances
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
2Na(s) + Cl₂(g) → 2NaCl(s).
mol Na :
\(\tt \dfrac{4.6}{23}=0.2\)
mol NaCl :
\(\tt \dfrac{11.7}{58,44 }=0.2\)
limiting reactant = Na(mol NaCl from mol Na, mol ratio : 2 : 2)
mol Cl₂:mol Na=2:1
mol Cl₂ =
\(\dfrac{1}{2}\times 0.2=0.1\)
mass Cl₂ :
MW Cl₂ = 71 g/mol
\(\tt 0.1\times 71=7.1~g\)
questionyou have two solutions. one is made of 100.0 g of methanol in 500.0 g of water. the other has 200.0 g of methanol in 500.0 g of water.which statement best describes which solution will have the smaller freezing-point depression?
Answers
To calculate which solution will have the smaller freezing-point depression between the two solutions, one with 100.0 g of methanol in 500.0 g of water and the other with 200.0 g of methanol in 500.0 g of water, we need to consider the concept of freezing point depression.
Freezing point depression is a phenomenon in which the freezing point of a solution is lower than that of the pure solvent. It depends on the concentration of the solute, in this case, methanol.
Solution 1: 100.0 g methanol in 500.0 g water
Solution 2: 200.0 g methanol in 500.0 g water
Comparing the two solutions, Solution 1 has a lower concentration of methanol than Solution 2. Therefore, Solution 1 will have a smaller freezing-point depression compared to Solution 2, since the freezing point depression is directly proportional to the concentration of the solute in the solution.
To know more about freesing point depression : https://brainly.com/question/31357864
#SPJ11
37)
What is NOT a way that plants affect landforms?
A)
by weathering rock with chemicals
B)
by weathering rock with their roots
by holding soil in place with their roots
D
by removing carbon dioxide from the air during photosynthesis
Answers
Answer:
dont know
Explanation:
what
ajjajaahhahahaahaahahqhqjqhwjahhahhqhqqhqhhqhahqah
11. What is the molarity of a solution that has 0.512 moles of NaCl dissolved in 0.24 L of solution? (Show WORK. Circle the correct answer: 2.1M or 1.2M)
Answers
Answer:
2.1 M
Explanation:
Molarity is moles per liter:
0.512 mol NaCl / 0.24 L = 2.1 M
Which one of the following forms of radiation can penetrate deepest into body tissue?
alpha
beta
gamma
positron
proton
Answers
Gamma radiation can penetrate the deepest into body tissue among the given forms of radiation.
Gamma radiation has the highest energy and smallest wavelength, which allows it to easily penetrate through the human body's soft tissues. Alpha particles, on the other hand, have a large size and low energy, which make them easily blocked by even a piece of paper. Beta particles are more energetic than alpha particles and can penetrate a few millimeters of human tissue, but not as much as gamma rays. Positrons have similar characteristics to electrons, which can penetrate a few centimeters of tissue, but still not as much as gamma radiation. Protons also have a limited penetration depth and are used for localized cancer treatment.
Learn more about tissue here:
https://brainly.com/question/17664886
#SPJ11
What is the measure for the amount of disorder in a system?
Answers
Answer: Entropy
Explanation: Entropy. A measure of the level of disorder of a system is entropy, represented by S
Hoped this helped!!!!
Sean is moving heavy furniture by sliding it
across the floor. He puts a blanket
underneath the furniture. Why did this help
him?
Answers
Answer:
the blanket let's him slide it across the floor because the has less friction with the floor than the furniture.
Answer:
Blanket underneath the furniture may reduce the kinetic friction
Explanation:
When sliding an object across a surface, the force of kinetic friction will oppose the movement of the object.
By placing a blanket between the object and the surface, the kinetic friction coefficient may be reduced.
Therefore, putting a blanket underneath the furniture may reduce the kinetic friction coefficient, then making it easier to slide the forniture.
(Hi fyi this answer took a lot of time & effort to make sure it was accurate, precise and to the point to be produced, so if you could opt this answer as the brainliest it would mean the world to me,)
Stay positive and Take care,
A fellow mate,
Cheers !
In which direction will the electrons be pulled in the bond between hydrogen and chlorine?
toward the chlorine atom
toward both atoms equally
sometimes toward chlorine and sometimes toward hydrogen
toward the hydrogen atom
Answers
Answer:
toward the hydrogen atom
Explanation:
Answer:
toward the chlorine atom.
Explanation:
took the quiz and got it correct :)
I need help with these questions

Answers
The definition of astronomic bodies are indicated below with the sentences defining them.
What are astronomic bodies?Astronomic bodies are celestial objects that occur naturally in space.
Here are their definitions below:
a. Supernova exhibits strong gravitational pull such that no light can escape
b. A nebula a large cloud of gas or dust in space.
c. A white dwarf is what a medium-mass star becomes at the end of it's life.
d. Protostar is the earliest stage of a star's life.
e. Black dwarf is a star left at the core of a planetary nebula.
f. Neutron stars are the remains of a high mass star.
g. A supernova is what occurs when a red supergiant star explodes.
Learn more about astronomic bodies at:
https://brainly.com/question/15434534
#SPJ1
(This is rly for science but there’s no category for that) Which statement best describes how scientists and engineers work together
in the research and development cycle?
O A. Engineers come up with scientific questions when they are
developing their design, and scientists do research to answer
them.
O B. Scientists test designs made by engineers and then use the
results to improve the designs.
C. Scientists develop a new technology, and then engineers test it by
doing experiments.
D. Engineers make a scientific discovery, and then scientists perform
research to verify it.
Answers
Answer:
A. Engineers come up with scientific questions when they are developing their design, and scientists do research to answer them.
PLEASE NO LINKS OR FILE SCAMS
what is the balanced equation for CuSO4 + 2NaOH → Cu(OH)2 + Na2SO4 I just want the answer please help
Answers
Let G and H be groups. Prove if φ(g) = eH for all g ∈ G, the map φ: G to H is a group homomorphism
Answers
φ(g1 * g2) = eH = φ(g1) * φ(g2).
This completes the proof that φ: G → H is a group homomorphism.
By showing that the map φ preserves the group operation, we have demonstrated that it is a group homomorphism.
To prove that φ: G → H is a group homomorphism, we need to show that it preserves the group operation. In other words, for any two elements g1 and g2 in G, φ(g1 * g2) = φ(g1) * φ(g2), where * denotes the group operation in G, and * denotes the group operation in H.
Given that φ(g) = eH for all g ∈ G, where eH is the identity element in H, we can start the proof as follows:
Let g1, g2 ∈ G. We want to show that φ(g1 * g2) = φ(g1) * φ(g2).
Since φ(g) = eH for all g ∈ G, we have φ(g1) = eH and φ(g2) = eH.
Now, consider the product g1 * g2 in G. Applying φ to both sides, we have:
φ(g1 * g2) = φ(g1) * φ(g2).
Substituting the values of φ(g1) and φ(g2), we get:
φ(g1 * g2) = eH * eH.
Since eH is the identity element in H, the product eH * eH is simply eH.
To know more about homomorphism
https://brainly.com/question/6111672
#SPJ11
pls help i’ve been doing exercises about it but i thing im just too tired to think rn

Answers
The number of moles to the equations are as follows;
1. 0.800 mol 2. 0.0814 mol 3. 0.162 mol
4. 2.8197 mol 5. 1.8798 mol 6. 2.8917 mol 7. 4mol of LiNO3 should make 2 mol of Li2SO4
How do we find the moles for the equation?1. 0.400 x (2/1) = 0.800 mole
2. 7.50(1/46.07) = 0.1628
0.1628(1/2) = 0.0814 mol
3. 7.50(1/46.07) = 0.1628
0.1628(2/2) = 0.1628 mol
4. 150(1/159.69) = 0.9399
0.9399(3/1) = 2.88197 mol
5. 150(1/159.69) = 0.9399
0.9399(2/1) = 1.898 mole
6. 0.9399(3/1) = 2.88197 mol
7. 250(1/109.94) = 2.2737 mole
The above answer is based on the information below, that was gotten from the picture;
Using the equation:
C6H12O6 → 2 C2H5OH + 2 CO2
6. How many moles of CO2 are produced when 0.400 mol of C6H12O6 react?
7. How many moles of C6H12O6 are needed to form 7.50 g of C2H5OH?
8. How many moles of CO2 form when 7.50 g of C2H5OH are produced?
Using the equation:
Fe2O3 + 3CO → 2 Fe + 3CO2
9. Calculate the number of moles of CO that can react with 150 g of Fe2O3.
10. Calculate the number of moles of Fe formed when 150 g of Fe2O3 reacts.
11. Calculate the number of moles of CO2 formed when 150 g of Fe2O3 reacts.
Using the equation:
Pb(SO4)2 + 4 LINO3 → Pb(NO3)4+2 Li2SO4
12. How many moles of lithium nitrate will be needed to make 250 grams of lithium sulfate?
Find more exercises on finding mole;
https://brainly.com/question/18265914
#SPJ1
if equal amounts of helium and argon are placed in a porous container and allowed to escape, which gas will escape faster and how much faster?
A: Argon is 3.16x faster
B: Helium is 0.316x faster
C: Argon is 0.316x faster
D: Helium is 3.16x faster
Answers
Answer:
B: Helium is 0.316x faster
If equal amounts of helium and argon are placed in a porous container and allowed to escape, helium will diffuse 3.16x faster than argon.
According to Graham's law of diffusion of gases, the rate of diffusion of a gas is inversely related to the square root of its molecular weight.
Mathematically, when two gases are being compared;
r1/r2 = √m2/√m1
Where r1 = diffusion rate of gas 1, r2 = difussion rate of gas 2, m1 = molar weight of gas 1, and m2 = molar weight of gas 2.
Molar weight of helium = 4
Molar weight of argon = 39.9
Hence,
r helium/r argon = √39.9/√4
= 6.317/2
= 3.16
Therefore, 3.16 x r argon = r helium.
In other words, helium will diffuse 3.16x faster than argon.
More on diffusion rate of gases can be found here: https://brainly.com/question/2967915
What volume of 0.530 M calcium hydroxide solution is required to titrate 35.0 mL of 0.440 M phosphoric acid to the third equivalence point?
Answers
The volume of calcium hydroxide solution required to titrate 35.0 mL of 0.440 M phosphoric acid to the third equivalence point is 87.5 mL.
In the given case, we have to calculate the volume of 0.530 M calcium hydroxide solution required to titrate 35.0 mL of 0.440 M phosphoric acid to the third equivalence point.
The chemical equation for the reaction of phosphoric acid with calcium hydroxide is given below:
H₃PO₄ + Ca(OH)₂ → Ca(H₂PO₄)₂ + 2H₂O
Molar mass of H₃PO₄ = 3(1.008) + 1(30.974) + 4(15.999)
= 98.0 g/mol
Molar mass of Ca(OH)₂ = 1(40.078) + 2(15.999)
= 74.1 g/mol
Number of moles of H₃PO₄ in 35.0 mL of 0.440 M
phosphoric acid= Molarity × Volume(in liters)
= 0.440 × (35.0/1000)
= 0.0154 mol
Number of moles of Ca(OH)₂ required to react completely with 0.0154 mol of H₃PO₄= 0.0154 mol
Number of moles of Ca(OH)₂ present in the solution
= Molarity × Volume(in liters)
= 0.530 × Volume
Calcium hydroxide will react with phosphoric acid until the third equivalence point is reached.
Ca(OH)₂ + 2H₃PO₄ → Ca(H₂PO₄)₂ + 2H₂O(moles)
0.0154 0.031 0.0464
(Molarity) 0.53 0.53 0.53
(Volume) V₁ V₂ V₃
Equivalence point is the point where the number of moles of two reactants is equal to each other.The number of moles of H₃PO₄ is equal to the number of moles of Ca(OH)₂ at the third equivalence point,
So, 0.0464 mol of Ca(OH)₂ is required to react completely with 0.0154 mol of H₃PO₄ .
Volume of 0.530 M calcium hydroxide solution required to titrate 35.0 mL of 0.440 M phosphoric acid to the third equivalence point
= (0.0464 mol)/(0.530 mol/L)
= 0.0875
L= 87.5 mL (rounded to three significant figures)
Therefore, the volume of 0.530 M calcium hydroxide solution required to titrate 35.0 mL of 0.440 M phosphoric acid to the third equivalence point is 87.5 mL.
Learn more about equivalence point :
brainly.com/question/31484109
#SPJ11
which of the following acids will have the strongest conjugate base?
A. CI⁻
B. CH₃COO⁻
C. SO₄⁻
D. NO₂⁻
Answers
Among the given options, the strength of the conjugate base depends on the acidity of the corresponding acid. The stronger the acid, the weaker its conjugate base will be.
In this case, we can assess the acidity of the acids by considering their molecular structures and the factors that influence acidity.
A. CI⁻ (chloride ion) is the conjugate base of hydrochloric acid (HCl), a strong acid. Since HCl is a strong acid, its conjugate base CI⁻ is very weak.
B. CH₃COO⁻ (acetate ion) is the conjugate base of acetic acid (CH₃COOH), which is a weak acid. Weak acids tend to have relatively stronger conjugate bases. Therefore, CH₃COO⁻ is stronger compared to CI⁻.
C. SO₄⁻ (sulfate ion) is the conjugate base of sulfuric acid (H₂SO₄), a strong acid. Similar to HCl, H₂SO₄ is a strong acid, resulting in a weak conjugate base, SO₄⁻.
D. NO₂⁻ (nitrite ion) is the conjugate base of nitrous acid (HNO₂), which is a weak acid. Therefore, NO₂⁻ would have a relatively stronger conjugate base compared to CI⁻ and SO₄⁻.
In conclusion, among the given options, CH₃COO⁻ (acetate ion) would have the strongest conjugate base.
Learn more about base here ; brainly.com/question/31939284
#SPJ11
Please Help! No weird answers please!
For some traits, you will notice that there are fewer people with dominant traits than with the recessive trait. If you were a geneticist just discovering this phenomenon, what would your explanation be?

Answers
Answer:
The relationship of genotype to phenotype is rarely as simple as the dominant and recessive ... Why can you possess traits neither of your parents have? ... so concluded that there were some traits that dominated over other inherited traits. ... to notice a variety of relationships between alleles that code for the same trait.
Which of the following statements is true about the relationships between photon energy, wavelength, and frequency?
Group of answer choices
The photon frequency is proportional to energy and inversely proportional to wavelength.
The photon frequency is inversely proportional to energy and proportional to wavelength.
The photon frequency is proportional to energy and proportional to wavelength.
The photon frequency is inversely proportional to energy and inversely proportional to wavelength.
Answers
Answer: The Answer is A.
Explanation:
The amount of energy is directly proportional to the photon's electromagnetic frequency and thus, equivalently, is inversely proportional to the wavelength. The higher the photon's frequency, the higher its energy. Equivalently, the longer the photon's wavelength, the lower its energy.
Hope this Helps!
The photon energy, wavelength, and frequency are the characteristic of the waves and particles. "The photon frequency is proportional to energy and inversely proportional to wavelength." Thus, option A is correct.
What is photon energy?Photon energy has been defined as the energy constituted by the photon of the atom. It is given by the product of Planck's constant and wave frequency. The frequency of the photons is in inverse relation to the wavelength. It is given as,
E = hυ = h c / λ
Here, E is energy, h is Planck's constant, c is the speed of light, υ id frequency, and λ is the wavelength.
On the other hand, the frequency is in direct relation to the energy. The higher the frequency of the photons higher will be its energy.
Therefore, option A. frequency is inversely proportional to the wavelength.
Learn more about photon energy, here:
https://brainly.com/question/2393994
#SPJ2
Which property is a physical property?
Question 5 options:
becomes moldy quickly
easy to digest
malleable (the quality of something that can be shaped into something else without breaking )
does not burn
Answers
Answer: The answer is malleable.
Explanation: Malleability is a physical property that some elements of matter have that can be broken down into sheets to give them a certain shape without breaking. This physical property belongs to plasticity. It is a characteristic that some metals have, sheets of said metal can be obtained. The heat needs to be increased, some examples are gold, platinum, zinc, tin, etc.
draw the structure of the aromatic product from the reaction shown. the starting material is a benzene ring with a hydroxy group on carbon 1 and an n h 2 on carbon 4. this reacts with one equivalent of acetic anhydride, which is an oxygen flanked by two carbonyls, each bonded to a methyl group.
Answers
The structure of the aromatic product is shown below: \(O=C-N-C_1=CH_2-C_2=C_3C_4=C(OH)C=C_3C=C_2 .\)
What is aromatic ?Aromatic molecules are a type of organic compound that contain carbon atoms connected by bonds known as double bonds. These molecules possess a distinct odor, or smell, and are known as aromatic compounds. They are often found in essential oils, perfumes, and food flavorings.
The reaction of a benzene ring with a hydroxy group on carbon 1 and an NH₂ on carbon 4 with one equivalent of acetic anhydride (which is an oxygen flanked by two carbonyls each bonded to a methyl group) produces an aromatic product. This product will be an amide, and it will have an oxygen double-bonded to a nitrogen, with the nitrogen also single-bonded to the carbon 4 of the benzene ring. The oxygen will also be single-bonded to the carbon 1 of the benzene ring. The two carbonyl groups of the acetic anhydride will each be single-bonded to a different carbon of the benzene ring.
To learn more about aromatic
https://brainly.com/question/30899828
#SPJ4
Here is a sample graph from Lesson 4.2 - Can you determine the general relationship between the percentage yield of ammonia and pressure?
Which temperature was the most beneficial for this experiment?
How many different “systems” were tested here?
Here’s a tough one -
Which was held “constant” during the test run? Select one.
a. The percentage yield of ammonia
b. Pressure
c. Temperature
d. All were constant
e. None were constant
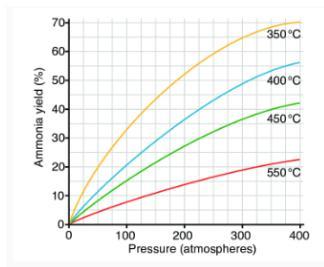
Answers
Option D is correct All remained the same Here, a variety of "systems" were put to the test.
Throughout an experiment, the control variable is constant. In an experiment, the independent variable is changed in an effort to change the experiment's dependent variable. In order to ensure that the effects on the dependent variable are definitely caused by changes in the independent variable, the control variable is kept constant.
The gas constant can be expressed using the values and units listed below. The values of the gas constant R are typically listed under "Physical Constants" in textbooks and handbooks because it is a constant that applies to all gases without exception. When the temperature remains constant, the pressure of a gas is inversely proportional to its volume. When the temperature is constant, the product of pressure and volume is constant. This relationship is known as Boyle's law or Mariette's law.
Learn more about variable from here;
https://brainly.com/question/30763805
#SPJ1
what mass of potassium sulfate is needed to make 2500.0ml of 2.0m solution?
(ANSWER THIS AND SHOW THE WORRRKKKKK, AND EXPLAINNN IT) THIS ISSS 60 POIUNTS PLZZ TAKE IT SERIOSLY
Answers
Molarity
Chemists primarily need the concentration of solutions to be expressed in a way that accounts for the number of particles that react according to a particular chemical equation. Since percentage measurements are based on either mass or volume, they are generally not useful for chemical reactions. A concentration unit based on moles is preferable. The molarity (M) of a solution is the number of moles of solute dissolved in one liter of solution. To calculate the molarity of a solution, you divide the moles of solute by the volume of the solution expressed in liters.
Note that the volume is in liters of solution and not liters of solvent. When a molarity is reported, the unit is the symbol M and is read as “molar”. For example a solution labeled as 1.5 M NH 3 is read as “1.5 molar ammonia solution”.
Sample Problem: Calculating Molarity
A solution is prepared by dissolving 42.23 g of NH 4 Cl into enough water to make 500.0 mL of solution. Calculate its molarity.
Step 1: List the known quantities and plan the problem.
& underline{text{Known}} &&underline{text{Unknown}} \& text{mass}=42.23 text{g} NH_4Cl && text{molarity}= ? text{ M}\& text{molar mass} NH_4Cl=53.50 text{g} / text{mol} \& text{volume solution}=500.0 text{mL}=0.5000 text{L}
The mass of the ammonium chloride is first converted to moles. Then the molarity is calculated by dividing by liters. Note the given volume has been converted to liters.
Step 2: Solve.
42.23 text{ g } NH_4Cl times frac{1 text{ mol } NH_4Cl}{53.50 text{ g } NH_4Cl} &= 0.7893 text{ mol } NH_4Cl\frac{0.7893 text{ mol } NH_4Cl}{0.5000 text{ L}} &= 1.579 text{ M}
Step 3: Think about your result.
The molarity is 1.579 M, meaning that a liter of the solution would contain 1.579 mol NH 4 Cl. Four significant figures are appropriate.
In a laboratory situation, a chemist must frequently prepare a given volume of solutions of a known molarity. The task is to calculate the mass of the solute that is necessary. The molarity equation can be rearranged to solve for moles, which can then be converted to grams. See sample problem 16.3.
Sample Problem:
A chemist needs to prepare 3.00 L of a 0.250 M solution of potassium permanganate (KMnO 4 ). What mass of KMnO 4 does she need to make the solution?
Step 1: List the known quantities and plan the problem.
Known
molarity = 0.250 M
volume = 3.00 L
molar mass KMnO 4 = 158.04 g/mol
Unknown
mass KMnO 4 = ? g
Moles of solute is calculated by multiplying molarity by liters. Then, moles is converted to grams.
Step 2: Solve.
text{mol KMnO}_4 = 0.250 text{ M KMnO}_4 times 3.00 text{ L} &= 0.750 text{ mol KMnO}_4\0.750 text{ mol KMnO}_4 times frac{158.04 text{ g KMnO}_4}{1 text{ mol KMnO}_4} &=119 text{ g KMnO}_4
Step 3: Think about your result.
When 119 g of potassium permanganate is dissolved into water to make 3.00 L of solution, the molarity is 0.250 M.
Laura has three beakers. Each contains 200 cm³ of a colourless liquid. Describe how Laura could find out which beakers contain pure water, and which contain solutions. Explain your answer.
Answers
Laura could use a few different methods to determine which beakers contain pure water and which contain solutions. One method is to test the boiling point of each liquid. Pure water boils at 100 degrees Celsius at standard pressure. If the liquid in a beaker boils at a temperature higher than 100 degrees Celsius, it is likely a solution and not pure water. Another method is to test the freezing point of each liquid. Pure water freezes at 0 degrees Celsius at standard pressure. If the liquid in a beaker freezes at a temperature other than 0 degrees Celsius, it is likely a solution and not pure water.
Another method is through density test. Pure water has a density of 1g/cm³ at 4°C. Laura can use a hydrometer, which is an instrument that measures the density of a liquid to check if the density of the liquids in the beakers is equal to 1g/cm³. If it is not, then it is not pure water.
Additionally, Laura could also test the conductivity of the liquids. Pure water is a poor conductor of electricity, whereas solutions can conduct electricity. Laura could use a conductivity meter to check the conductivity of the liquids. If a liquid conducts electricity, then it is likely a solution and not pure water.
Finally, Laura could also use a refractometer, which measures the refractive index of the liquid. The refractive index of pure water is 1.333 and any deviation from this value indicates the presence of dissolved solutes.
It's important to notice that no single test can confirm that a liquid is pure water, but a combination of tests can give us a strong indication of it.