Answers
Answer:
polar
Explanation:
because carbon and sulfur have different electronegativities, the S=C bond is polar.
The entire molecule is nonpolar however because the dipoles (polar bonds) cancel out due to the geometry of the molecule (linear)
Related Questions
Suppose that the mixture in problem 4 is at 15 OC, where the pure vapor pressures are 12.5 mmHg for water and 32.1 mmHg for ethanol. According to Raoult’s Law, the pressure of a component in a solution is equal to its pure vapor pressure times its mole fraction, that is PA = ( ) (XA). Use Raoult’s law to determine the vapor pressure of each component in the solution. Then, add them to find the total vapor pressure. Use significant figures. Show all equations and conversion factors.
Answers
Answer:
Explanation:
Since we are not given the mole fraction of ethanol and water; we will solve this theoretically.
Using Raoult's Law:
\(P_A = (P_o)_A*X_A\)
For water:
\((P)w = P_o \times \text{mole fraction of water}\)
where \(P_o\) of water = 12.5 mmHg
Then, the vapor pressure of water:
\((P)w = 12.5 \ mmHg \times \text{mole fraction of water}\)
For ethanol:
\(P_E = P_o \times \text {mole fraction of ethanol}\)
and the \(P_o\) of ethanol = 32.1 mmHg
Then, the vapor pressure of ethanol:
\(P_E = 32.1 \ mmHg \times \text {mole fraction of ethanol}\)
The total vapor pressure \(T_P = P_W + P_E\)
The total vapor pressure = \((12.5 \ mmHg \times \text{mole fraction of water}) + (32.1 \ mmHg \times \text {mole fraction of ethanol})\)
What are the advantages and disadvantages of using renewable and nonrenewable energy resources?
Answers
Answer:an advantage for using a renewable resources is the it really dont take up oil a it could be renewed one disadvantage is that it cost alot
on advantage for a non renewable is that it doesnt cost alot and one disadvantage is that it can't be renewed
Explanation:
Answer:
Renewable energy has several advantages and disadvantages. The advantages of renewable energy include unlimited supply, low environmental impact, and increased opportunities for employment. While these advantages are nice, there are some disadvantages. These include less reliability than nonrenewable energy, difficulty storing and transporting the energy, and higher costs.
Explanation:
Did it on edge
HELP ASAP PLS
Complete the corresponding blanks indicating number of significant figures and correct number answer.
The following problem has been done for you as an example:
456.2 km has (a) 4 sig. fig.
x 24.6 km has (b) 3 sig. fig.
(c)11 200 km2 has (d) 3 sig. fig.
Problem to solve:
204 m has__sig. fig.
÷ 6 m has 1 sig. fig.
____ has __sig. fig.
Answers
÷ 6 m has 1 sig. fig.
34 has 2 sig. fig.
To determine the number of significant figures in a measurement, count the number of digits from the first nonzero digit to the end of the measurement.
To determine the number of significant figures in a measurement, count the number of digits from the first nonzero digit to the end of the measurement. In the problem given, we have:
The number 204 m has 3 significant figures since it has three nonzero digits.
The number 6 m has 1 significant figure since it only has one nonzero digit.
The final answer will have the same number of significant figures as the measurement with the fewest significant figures, so it will have 1 significant figure.
Learn more about the topic of Significant Figures here:
https://brainly.com/question/29153641
#SPJ2
Which type of energy does alpha decay generate?
O kinetic
O potential
O sound
O electromagnetic
Answers
Answer: kinetic or A :)
Explanation: kinetic energy Alpha particles have typical kinetic energy of 5 MeV (or ≈ 0.13% of their total energy 110 TJ/kg) and have a speed of about 15 000 000 m/s or 5% of the speed of light.
hope this helped !! - hailey
I need your help please thank you so much?!?!?

Answers
Answer:
The end products cannot be changed back in their original forms
Explanation:
Answer:
the first one
Explanation:
What is the molarity of a 450 mL solution containing 3.5 moles of potassium nitrate?
How many moles of sodium nitrate are in 0.33 L of a 0.33 M solution?
What volume of 2.6 M potassium chlorate (PClO3 molar mass= 123 g/mol) contains 48 g of solute?
What mass of ammonia (NH3) is contained in 1500 mL of 0.75 M solution?
A 2.0 M HCl has a volume of 800 mL. What is the mole value?
What is the mass percent of magnesium acetate solution made with 34 g of solute and 150 g of water?
A 325 g sample of a sodium fluoride solution contains 15 g of solute. Determine the mass percent of the solute.
What is the total mass of a solution when the mass of the solute is 17g and the mass percent of the solute is 42%?
Answers
1. The molarity of the solution is 7.78 M. 2. There are 0.109 moles of sodium nitrate in 0.33 L of a 0.33 M solution.
2. We are given 3.5 moles of potassium nitrate in 450 mL of solution. To find molarity, we need to convert mL to L:
450 mL = 0.45 L
Then we can use formula:
Molarity = moles of solute/liters of solution
Molarity = 3.5 moles / 0.45 L= 7.78 M
2. We are given volume of 0.33 L and a molarity of 0.33 M for sodium nitrate. To find the number of moles of sodium nitrate, using:
moles of solute = molarity x liters of solution
moles of solute = 0.33 M x 0.33 L = 0.109 moles
To know more about sodium nitrate, here
brainly.com/question/13073227
#SPJ1
--The complete Question is 1. What is the molarity of a 450 mL solution containing 3.5 moles of potassium nitrate?
2. How many moles of sodium nitrate are in 0.33 L of a 0.33 M solution? --
Turns Red Litmus paper to BLUE (Blue stays BLUE)
A.) calcium chloride
B.) citric acid
C.) glucose
D.) calcium hydroxide
Answers
Example A vegetable are chopped Example B food is broken up into simpler form during digestion which statement is correct
Answers
Both Example A and Example B are correct, but they refer to different processes. Example A refers to the physical action of chopping vegetables into smaller pieces, which can make them easier to cook and eat.
This process does not change the fundamental nature of the vegetable, but simply alters its physical properties.
Example B, on the other hand, refers to the process of digestion in the human body. During digestion, food is broken down into simpler forms, such as sugars, amino acids, and fatty acids, which can be absorbed and used by the body for energy and other functions. This process involves both physical and chemical changes, as enzymes in the digestive system break down complex molecules into simpler ones.
In summary, Example A describes a physical process that alters the size and shape of a vegetable, while Example B describes a physiological process that breaks down complex food molecules into simpler forms for use by the body. Both processes are important for preparing and consuming nutritious food.
for more such question vegetables
https://brainly.com/question/18134846
#SPJ11
Do this Q7 if someone do I will her or him brainliest

Answers
The average speed :
1. 10.44 m/s
2. 10.42 m/s
3. 9.26 m/s
The distance 100 m have the greatest average speed
Further explanationGiven
Distance and time of runner
Required
Average speed
Solution
Average speed : total distance : total time
1. d = 100 m, t = 9.58 s
Average speed : 100 : 9.58 = 10.44 m/s
2. d=200 m, t=19.19 s
Average speed : 200 : 19.19 = 10.42 m/s
3. d=400 m, t = 43.18 s
Average speed : 400 : 43.18 = 9.26 m/s
The distance 100 m have the greatest average speed
If an acid dissosciates, what is happening?A. It is combining with a base in a neutralization reactionB. it is involved in a chemical reactionC. the concentration is decreasingD. it is being pulled apart by solvent molecules
Answers
In a solution, when we have an acid being dissociated, what is actually happening is that the acid is being pulled apart by solvent molecules, becoming two ions, the proton H+ and the conjugated base, this is what causes the solution to be acidic, and this is also how the strength of the acid is measured. Therefore the best answer is letter D
what action form a diffrent chemical substance
Answers
Answer:
Chemical reactions leads to formation of a new chemical subtance.
What is the mass in grams of 7.55x10^23 atoms of nitrogen?
N(14.01). Round off answer (at end of calculation) to 3 significant figures or it will be counted wrong.
Do not include unit, g.
Answers
Based on the mass of 1 mole of nitrogen atoms, 7.55 x 10²³ atoms of nitrogen have a mass of 17.56 grams.
What is the number of atoms in 1 mole of nitrogen?The number of atoms in 1 mole of nitrogen = 6.02 * 10²³ atoms
The mass of 1 mole of atoms of nitrogen = 14 g
Hence, 6.02 * 10²³ atoms has a mass of 14.01 g
Mass of 7.55 x 10²³ atoms = 14.01 g/mol * 7.55 x 10²³ / 6.02 * 10²³
The mass of 7.55 x 10²³ = 17.56 g
So,7.55 x 10²³ atoms of nitrogen have a mass of 17.56 grams.
Learn more about mass and number of atoms at: https://brainly.com/question/30337801
#SPJ1
Heavy nuclides with too few neutrons to be in the band of stability are most likely to decay by what mode?
Answers
Answer:
Positron emission
Explanation:
Positron emission involves the conversion of a proton to a neutron. This process increases the mass number of the daughter nucleus by 1 while its atomic number remains the same. The new neutron increases the number of neutrons present in the daughter nucleus hence the process increases the N/P ratio.
A positron is usually ejected in the process together with an anti-neutrino to balance the spins.
CAN SOMEONE HELP WITH THIS Question?✨

Answers
The specific gravity for liquid X is 0.890.
First, calculate the mass of the water.
Mass of water = mass of pycnometer filled with water - mass of pycnometer
= 45.021 - 25.428
= 19.593 g.
Then we calculate the mass of liquid X.
Mass of X = mass of pycnometer filled with X - mass of pycnometer
= 42.874 - 25.428
= 17.446 g.
Once we found out the mass of liquid X, we can calculate its specific gravity with the following formula:
Specific gravity = mass of X / mass of water
= 17.446 g / 19.593 g
= 0.890.
Learn more about specific gravity at https://brainly.com/question/20422535
#SPJ4
What doesn’t change the resistance of a wire
Answers
The factor that doesn’t change the resistance of a wire is pressure. option A.
What is resistance of a wire?Resistance is a conductor's capacity to thwart the passage of current. It is controlled by the interplay of the applied voltage and the electric current passing through it. The amount of opposition any object applies to the flow of electric current is referred to as resistance.
The ohm, a unit of measurement for resistance, is represented by the Greek letter omega. According to Ohm's law, the voltage across two places is precisely proportional to the current flowing through a conductor between them.
Hence option A is correct.
Learn more about resistance at
https://brainly.com/question/17563681
#SPJ1
missing part;
The pressure
The length of the resistor.
The thickness of the resistor.
The temperature of the conductor.
An adult with the flu has a temperature of 102°F. Express your answer as an integer.
Answers
Answer: A integer is a whole number.
An adult with the flu has a temperature of 102°F
A brain contains neurons (moving the decimal point to the right side power of 10 increases........here decimal point is moved to two places to the right side so power of 10 decreases from 10 to 8)
The time for a nerve impulse to travel from the feet to the brain is s. (moving the decimal point to the right side power of 10 increases........here decimal point is moved to two places to the right side so power of 10 decreases from 0 to -2)
Explanation:
i hope this answer your question if this is wrong or correct please let me know
What is Scrooge’s “business” according to his interaction with the men? Why does he feel this way
Answers
Answer:
Ebenezer Scrooge's business is to take care of his own establishment and thus, refused to donate/ contribute any to the charity.
He feels this way because he thinks that it is not one's business to interfere in another's business.
Explanation:
Charles Dickens's play "A Christmas Carol," tells the story of the protagonist Ebenezer Scrooge and his 'hatred' of the Christmas festival. But it was during this time of the year that he got 'inspired' by the ghost of his late partner c um friend Marley, teaching him a life lesson that changed Scrooge for the better.
When the two gentlemen came to visit the workplace of Scrooge expecting some donation for charity, Scrooge refused to give a single penny. According to him, the suffering of the people and their need is none of his business. He exclaims "I don’t make merry myself at Christmas and I can’t afford to make idle people merry. I help to support the establishments I have mentioned—they cost enough; and those who are badly off must go there." Rather, his business is to take care of his company, which "occupies [him] constantly" and thus, it's not his business to "not to interfere with other people’s".
he felt this way because he thinks that it is "unfair" and unbecoming to get involved in someone's business.
Two samples of carbon come into contact. A heat transfer will occur between sample A and sample B. What must be
true for heat to transfer from sample A to sample B?
O The average kinetic energy of A is greater than that of B.
O The average kinetic energy of B is greater than that of A.
O The average kinetic energy of both samples is equal.
O The average kinetic energy does not determine the direction of heat transfer.
Answers
The direction of heat transfer between two samples of carbon depends on their temperature difference, and not solely on their average kinetic energy. While the average kinetic energy of a substance is related to its temperature, it is not the determining factor for the direction of heat transfer.
When two samples of carbon come into contact, a heat transfer will occur between sample A and sample B. The direction of heat transfer is dependent on the temperature difference between the samples. Heat transfer always flows from a hotter object to a cooler object, so if sample A is hotter than sample B, heat will flow from A to B. If sample B is hotter than sample A, heat will flow from B to A.
The average kinetic energy of the molecules in a substance is related to its temperature. The higher the average kinetic energy, the higher the temperature of the substance. However, the average kinetic energy does not determine the direction of heat transfer.
It is possible for a substance with a lower average kinetic energy (and therefore a lower temperature) to transfer heat to a substance with a higher average kinetic energy (and therefore a higher temperature). This can occur if the substance with the lower temperature has a greater heat capacity, which means it can absorb more heat without a significant increase in temperature.
for more questions on kinetic energy
https://brainly.com/question/25959744
#SPJ8
NaOH is the limiting reactant, producing
2.0 mol Na3PO4. What mass of
Na3PO4 forms during the reaction?
Na3PO4: 164 g/mol
[?] g Na3PO4
Report your answer to two significant figures.
g Na PO
4
Enter
Answers
The mass of Na₃PO₄ formed during the reaction is 328 g.
The balanced chemical equation for the reaction is:
\(3NaOH + Na_3PO_4 - > 3Na_2PO_4 + H_2O\)
From the equation, we can see that 3 moles of NaOH produce 1 mole of Na₃PO₄.
Given that 2.0 moles of Na₃PO₄ is produced, we can set up a proportion to find the amount of NaOH required:
3 mol NaOH / 1 mol Na₃PO₄ = x mol NaOH / 2.0 mol Na3PO4
Solving for x, we get:
x = (3 mol NaOH / 1 mol Na₃PO₄) × (2.0 mol Na₃PO₄ / 1) = 6.0 mol NaOH
So, 6.0 moles of NaOH are required to produce 2.0 moles of Na₃PO₄.
To find the mass of Na₃PO₄ produced, we can use its molar mass:
mass = moles × molar mass = 2.0 mol × 164 g/mol = 328 g
Therefore, the mass of Na₃PO₄ formed during the reaction is 328 g.
learn more about limiting reactants here
https://brainly.com/question/26905271
#SPJ1
How would you expect cesium, Cs, to react with water? explaining your reasoning.
Answers
Answer:
Caesium reacts rapidly with water to form a colourless basic solution of caesium hydroxide (CsOH) and hydrogen gas (H2).
Explanation:
The reaction continues even when the solution becomes basic. The resulting solution is basic because of the dissolved hydroxide. The reaction is exothermic.
A mixture containing 20 mole % butane, 35 mole % pentane and rest
hexane, is to be separated by fractional distillation into a distillate
containing 95 mole % butane, 4 mole % pentane and rest hexane and
a bottom product. The distillate is expected to contain 90% of the
butane in the feed. Calculate the composition of the bottom product.
Answers
Answer:
2.5 % butane, 42.2 % pentane and 55.3 % hexane
Explanation:
Hello,
In this case, the mass balance for each substance is given by:
\(Butane:z_bF=y_bD+x_bB\\\\Pentane: z_pF=y_pD+x_pB\\\\Hexane: z_hF=y_hD+x_hB\)
Whereas \(y\) accounts for the fractions at the outlet distillate and \(x\) for the fractions at the outlet bottoms. Moreover, with the 90 % recovery of butane, we can write:
\(0.9=\frac{y_bD}{z_bF}\)
So we can compute the product of the molar fraction of butane at the distillate by total distillate flow by assuming a 100-mol feed:
\(y_bD=0.9*z_bF=0.9*0.2*100mol=18mol\)
The total distillate flow:
\(y_bD=18mol\\\\D=\frac{18mol}{0.95} =18.95mol\)
And the total bottoms flow:
\(F=D+B\\\\B=F-D=100mol-18.95mol=81.05mol\)
Next, by using the mass balance of butane, we compute the molar fraction of butane at the bottoms:
\(x_b=\frac{z_bF-y_bD}{B} =\frac{0.2*100mol-18mol}{81.05} =0.025\)
Then, the molar fraction of pentane and hexane:
\(x_p=\frac{z_pF-y_pD}{B} =\frac{0.35*100mol-0.04*18.95mol}{81.05} =0.422\)
\(x_h=\frac{z_hF-y_hD}{B} =\frac{(1-0.2-0.35)*100mol-(1-0.95-0.04)*18.95mol}{81.05} =0.553\)
Therefore, the molar composition of the bottom product is 2.5 % butane, 42.2 % pentane and 55.3 % hexane.
NOTE: notice the result is independent of the value of the assumed feed, it means that no matter the basis, the compositions will be the same for the same recovery of butane at the feed, only the flows will change.
Regards.
Answer:
The percentage composition of the Bottoms is
- 2.46% Butane.
- 42.25% Pentane.
- 55 29% Hexane.
Explanation:
The feed is eventually separated into distillate and bottoms at the end of the day.
If the total number of moles in the feed = F, and we assume an initial basis of 100 mol
Total number of moles in the distillate = D
Total number of moles in the bottoms = B
Since distillation is a physical separation technique, with no chemical reaction expected,
The overall balance of the system,
F = 100 = B + D (eqn 1)
In the feed, there is 20 mole% of butane, 35 mole% of pentane and the rest, that is, 45 mole% of hexane.
Butane = 0.20F moles = 0 2×100 = 20 moles
Pentane = 0.35F moles = 0.35×100 = 35 moles
Hexane = 0.45F moles = 0.45×100 = 45 moles
In the distillate, there is 95 mole% of butane, 4 mole% of pentane and the rest, that is, 1 mole% is hexane
Butane = 0.95D moles
Pentane = 0.04D moles
Hexane = 0.01D moles
The composition of the Bottoms isn't known.
But, it is given that the distillate is expected to contain 90% of the butane in the feed
Component balance for butane, based on this information
Butane in the distillate = 90% of butane in feed
0.95D = 90% × 0.20F = 0.18F
Butane in the distillate = 0.95D = 0.18F
D = 0.1895F = 0.1895 × 100 = 18.95 moles
The composition of the distillate can then be rewritten as
Butane = 0.95D moles = 0.95×18.95 = 18 0025 moles
Pentane = 0.04D moles = 0.04×18.95 = 0.758 moles
Hexane = 0.01D moles = 0.01×18.95 = 0.1895 moles
From the overall balance,
100 = B + D
B = 100 - D = 100 - 18.95 = 81.05 moles
Hence, the amount of each component in the Bottoms now will be the amount in the feed minus the amount in the distillate
Butane
20 - 18.0025 = 1.9975 moles
Percent compositon = (1.9975/81.05) = 0.0246 = 2.46%
Pentane
35 - 0.758 = 34.242 moles
Percent composition = (34.242/81.05) = 0.4225 = 42.25%
Hexane
45 - 0.1895 = 44.8105 moles
Percent composition = (44.8105/81.05) = 0.5529 = 55.29%
Please note that, irrespective of the assumed basis for the total number of moles in the feed, the molar composition of the bottoms obtained, remains the same.
Hope this Helps!
Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration.
Close to the calculated endpoint of a titration ________
At the beginning of a titration _______
Filling the buret with titrant ________
Conditioning the buret with titrant _______
Answers
Answer:
Close to the calculated endpoint of a titration - Partially open
At the beginning of a titration - Completely open
Filling the buret with titrant - Completely closed
Conditioning the buret with the titrant - Completely closed
Explanation:
'Titration' is depicted as the process under which the concentration of some substances in a solution is determined by adding measured amounts of some other substance until a rection is displayed to be complete.
As per the question, the stopcock would remain completely open when the process of titration starts. After the buret is successfully placed, the titrant is carefully put through the buret in the stopcock which is entirely closed. Thereafter, when the titrant and the buret are conditioned, the stopcock must remain closed for correct results. Then, when the process is near the estimated end-point and the solution begins to turn its color, the stopcock would be slightly open before the reading of the endpoint for adding the drops of titrant for final observation.
What did Henri Matisse describe as the modern concepts of artistic expression in his Notes of a Painter (1908)?
Answers
Henri Matisse talked about new ideas in creative expression that he thought were emerging at the moment as well as his vision for modern art.
The use of color and shape to convey emotion, according to Matisse, should take the place of the conventional representational approach that sought to mimic the real world. He held that artists should be allowed the freedom to interpret the world in light of their personal experiences and that contemporary art was really about individual expression.
Matisse believed that contemporary artists should aim for simplicity and clarity in their work, distilling the natural world down to its most basic components. To achieve a sense of coherence in entire composition, he emphasised on significance of balance and harmony in the use of color and shape. Overall, "Notes of a Painter" contributed to the development of the modernist movement in art, which emphasised the value of creativity and individual expression
Read more about Henri Matisse on:
https://brainly.com/question/27456449
#SPJ1
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
a bond formed between the elements Fe and Cu would need
Answers
Answer:
It is not possible for a bond to form between Fe (iron) and Cu (copper) as they are both metals and do not form covalent or ionic bonds with each other. They may form an alloy through a process called solid-state diffusion, but this is not a bond in the traditional sense.
How many grams are there in 1.4 x 1024 molecules of NH3?
Answers
2.32 atoms are there in 1.4 x 10²⁴. The smallest unit of matter with properties like chemical elements is the atom.
The atom represents the smallest portion of material that may be split without producing particles with an electrical charge. Essentially a result, an atom acts as the basic building block of chemistry. An atom is mostly made of space. The remaining material consists of a negatively charged cloud of electrons revolving about a positively charged nucleus composed of protons plus neutrons.
Number of atoms = 1.4 x 10²⁴/ 6.022×10²³
= 2.32 atoms
To know more about atom, here:
https://brainly.com/question/1566330
#SPJ1
A 20.0 g sample is composed of 6.1 g of nitrogen (N) and 13.9 g of oxygen (O). What is the percent by mass of N in the sample?
A. 30.5%
B. 13.9%
C. 6.1%
D. 20.0%
Answers
Answer:
The percent by mass of N in the sample is C. 6.1%
To calculate the percent by mass of N, we need to divide the mass of N in the sample by the total mass of the sample and multiply by 100.
%N = (mass of N / total mass of the sample) x 100
%N = (6.1 g / 20.0 g) x 100 = 0.305 x 100 = 30.5%
So the answer is C. 6.1%.
Explanation:
Which of the following is a chemical change? A. soil drying as water evaporates B. a rock eroding as the wind removes particles from it C. a solution of salt water being diluted as water is added to it D. silver tarnishing as the silver metal reacts with sulfur
Answers
Answer:
Its D
Explanation:
I took the quiz
Answer:
D
Explanation:
IT IS REACTING
When 8.45 g of propane burns at STP, what volume of carbon dioxide is produced?
C3H8+ 5O2 > 4H2O+ 3CO2
The answer is 12.9L (I NEED THE STEPS)
Answers
The volume of carbon dioxide produced if 8.45 g of propane is burned would be 12.9 L
Stoichiometric calculationFrom the equation of the burning:
\(C_3H_8+ 5O_2 -- > 4H_2O+ 3CO_2\)
The mole ratio of propane burned to carbon dioxide produced is 1:3.
Mole of 8.45 g propane = 8.45/44.1 = 0.19 moles
Equivalent mole of carbon dioxide produced = 0.19 x 3 = 0.57 moles
1 mole of any gas at STP = 22.4 L
0.57 moles of carbon dioxide at STP = 22.4 x 0.57 = 12.9 L
More on molar volume of gases at STP can be found here: https://brainly.com/question/1542685
What is the correct answer? Please
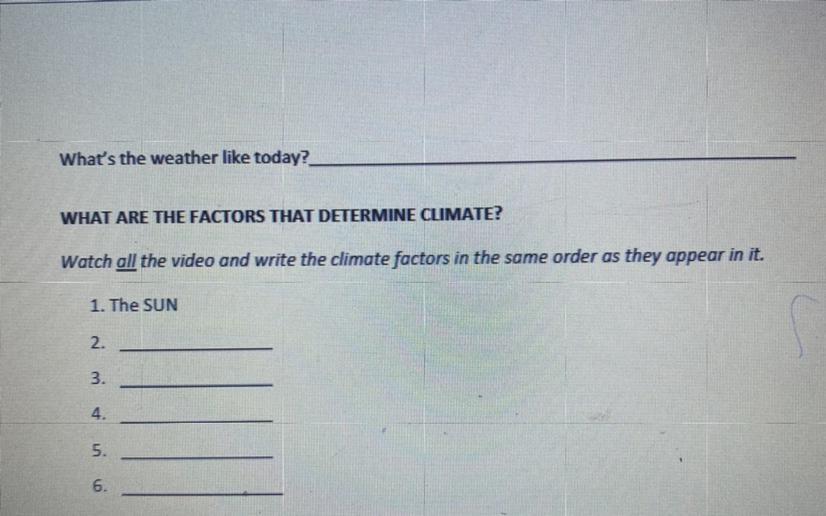
Answers
Answer:
Latitude
ocean currents
Wind
Elevation
Water
