Answers
The lead(II) nitrate solution has a molarity of 1.2M if 22.00 mL of 2.00 M potassium iodide is required to equilibrate with 18.00 mL of lead(II) nitrate.
The following formula can be used to determine a solution's molarity:
na/nb = CaVa/CbVb
Where;
Ca is the acid concentration.
Cb = base concentration
Va = acid volume
Vb = base volume
na = number of acid moles
nb = number of base moles
The reaction's balanced equation is as follows:
2KNO3 (aq) + PbI2 Pb(NO3)2 (aq) + 2KI (s)
22 × 2/18 × Cb = 2/1
44/18Cb = 2
Cb = 44 ÷ 36
Cb = 1.2M
Since 22.00 mL of 2.00 M potassium iodide is required to equilibrate with 18.00 mL of lead (II) nitrate, the lead(II) nitrate solution's molarity is 1.2M.
To learn more about molarity please click on below link
https://brainly.com/question/27133333
#SPJ4
Related Questions
A photon has a frequency of 5.40 × 10^4 Hz. Calculate the energy (in joules) of 1 mole of photons with this frequency. Enter your answer in scientific notation.
Answers
The energy (in joules) of 1 mole of photons with this frequency is 1.99 x 10⁻¹⁴ J per photon.
What is photon ?Should a substance happen to have a lot of electrons in a higher level, and a lower level is mostly empty then a photon can cause an electron to transfer from a higher state to a lower one. This change releases energy and creates a new photon, in addition to the one which caused the transfer. This photon can in turn induce more electrons to fall to a lower state.
use formula
The energy of 1 mole of photons with the given frequency can be calculated using the following equation:
Energy (J) = Avogadro's number x Plank's Constant x Frequency
Therefore,
Energy (J) = 6.02 x 10²³ x 6.626 x 10⁻³⁴ x 5.40 x 10⁴
Energy (J) = 1.99 x 10⁻¹⁴ J
Therefore, the energy of 1 mole of photons with a frequency of 5.40 x 10⁴ Hz is 1.99 x 10⁻¹⁴ J, expressed in scientific notation.
To know more about photon , visit ;
brainly.com/question/20912241
#SPJ1
A vial of Ancef 1 g is reconstituted with 5 mL of normal saline to yield 125mg / m * L How many mL of the medication should be given if a patient is prescribed 250 mg of the medication?
Answers
Ancef (Cefazolin) is a first-generation cephalosporin antibiotic that is used to treat bacterial infections. Cefazolin is available in several formulations, including injectable, intravenous, and powder for injection.
A vial of Ancef 1 g is reconstituted with 5 mL of normal saline to yield 125mg / m * L. We need to determine how many milliliters of the medication should be given if a patient is prescribed 250 mg of the medication.To begin with, let us first calculate the concentration of the reconstituted solution using the given data.1 gram of Ancef (Cefazolin) = 1000 milligrams (mg)5 mL of normal saline = 5000 milligrams (mg)Therefore, the total volume of the reconstituted solution = 5 mL + the volume of Ancef (Cefazolin)1 g of Ancef (Cefazolin) = 125 mg/mL (Given)Therefore, the volume of Ancef (Cefazolin) = (250 mg)/(125 mg/mL) = 2 mLTherefore, the total volume of the reconstituted solution = 5 mL + 2 mL = 7 mLThus, the amount of medication that should be given to the patient is 2 mL.For such more question on antibiotic
https://brainly.com/question/11849121
#SPJ8
What is the numerical value of the rate constant for a reaction in which [A] is reduced from 0.380 M to 0.215 M in a span of 26.3 s? Assume the units of the rate constant are s-1. Please enter your response to three of significant figures using E notation. For example, to report 1347.54 to three significant figures, type: 1.35E3
Answers
Answer:
k = 2.17E-2 s-1
Explanation:
Initial Concentration [A]o = 0.380 M
Final Concentration [A] = 0.215 M
time, t = 26.3 s
Unit of rate constant = s-1
Rate constant = ?
The units of the rate constant depends on the order of the reaction. This is a first order reaction due to the units.
The integral rate law for a first order reaction is;
ln[A] = ln[A]o - kt
ln(0.215) = ln(0.380) - k(26.3)
ln(0.215) - ln(0.380) = - k(26.3)
- k(26.3) = -0.57
k = 0.57 / 26.3 = 0.02167
Reporting to three significant figures using E notation;
k = 2.17E-2 s-1
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
which of the following are examples of single replacement reactions select all that apply.
1) Ca(OH)2(aq) + 2 HCl(aq) ----> CaCl2(aq) + 2 H2O(l)
2)Mg(s) + Zn(NO3)2(aq) ------> Mg(NO3)2(aq) + Zn(s) 3)Na2S(aq) + Cd(NO3)2(aq) ----> 2 NaNO3(aq)
4) K(s) + 2HCl(aq) ----> 2KCl (aq) +H2(g)
Answers
Answer:
4
Explanation:
please mark brainiest
K(s) + 2HCl(aq) ----> 2KCl (aq) +H2(g) is an example of single replacement reactions.
What do you mean single replacement reactions?A single-displacement reaction, also known as single replacement reaction or exchange reaction, is a chemical reaction in which one element is replaced by another in a compound.
Single-replacement reactions always involve two pure elements and one aqueous compound/solution. In the above reaction, the A and C would be pure elements.
A single replacement reaction, sometimes called a single displacement reaction, is a reaction in which one element is substituted for another element in a compound.
Learn more about single replacement reactions:
https://brainly.com/question/13903783
#SPJ2
A student collected a sample of a gas in a 165 mL gas bulb until it’s pressure was 765 mm Hg at a temperature of 27.0 Degrees C. If the sample had a mass of 0.452 g, what is the molar mass of the gas?
Answers
The molar mass of the given gas is equal to 68.48 g.
What is the ideal gas equation?The ideal gas law can be described as an equation of the state of a hypothetical perfect gas. This equation can be represented as the product of the volume and pressure of one-mole perfect gas is equal to the product of the universal gas constant and absolute temperature of that gas.
The mathematically, ideal gas equation can be written as follows:
PV = nRT
Where n is the moles of gas, P is the pressure, V is the volume of the gas, and R is the gas constant.
Given, the volume of collected gas, V = 165 ml = 0.165 L
The temperature of the gas, T = 27° C = 273 + 27 = 300 K,
The pressure of gas, P = 765 mmHg = 0.99 atm
The value of the gas constant, R = 0.082 atm L /K mol
Substituting the values V, R, P, and T in the equation, we get:
The number of moles of the gas, n = PV/RT
n = 0.99 ×0.165/(0.082 × 300)
n = 0.0066 mol
The molar mass of the gas = 0.452/ 0.0066 =68.48 g/mol
Learn more about the ideal gas equation, here:
brainly.com/question/3637553
#SPJ1
What type of reaction will occur if AH is negative and entropy increases?
O spontaneous reaction
O Gibbs free reaction
O exothermic reaction
endothermic reaction
Answers
The type of reaction that will occur if AH is negative and entropy increases is option A which is a spontaneous reaction .
Spontaneous reaction explained.The spontaneity of any chemical reaction depends on the Gibbs free reaction which is related to entropy change and enthalpy change.ΔG= ΔH-TΔS
Where T is temperature change in kelvin.If AH is negative, entropy will increases which is ΔS. Then the ΔG will depend on the temperature change.
If TΔS is larger than ΔH, the reaction will be spontaneous and ΔG will be negative which means the reaction will move forward without any external input.
When a change in entropy increases with the temperature in the system, the reaction will be spontaneous and this will make the Gibbs free energy to be negative.
Learn more about spontaneous reaction below.
https://brainly.com/question/14061406
#SPJ1
Complete each row of the table below by filling in the missing pre
1 mol
1 M mol
1 m mol
1
mol
A
ha
10
10
= 10
= 10
0
0
-1
mol
mol
mol
mol
Н
X
5

Answers
Complete of row:
0.01 mol = 10⁻² mol1 M mol = 10³ mol1 m mol = 10⁻³ mol0,1 mol = 10⁻¹ molA mole is a unit of account for chemistry. The unit of account is used to facilitate the calculation of an object.
One mole of any substance will have the same number of particles, which is equal to 6.02 × 10²³ particles." For example, 1 mole of air contains 6.02 × 10²³ molecules of H₂O. 1 mole of oxygen gas contains 6.02 × 10²³ molecules of O.
The mole scale is needed as an indication of the amount of substance or compound, mole is the gram of substance divided by the relative molecular mass (Mr). The formula for calculating the moles of a compound is n = gram/Mr, in this case, n is the moles of the substance and gr is the mass of the substance.
Learn more about mole at https://brainly.com/question/29367909
#SPJ1
Two samples A and B of chloride of
gold contains 15.1% and 35.1% chloride
respectively show that these figures are
in agreement with the law of multiple
proportion [Au=197, cl-35.5)
Answers
The composition of the atoms in the compounds are in simple ratio to each other, they obey the law of multiple proportion.
From the information provided in the question;
A contains 15.1% of Cl and 84.9% of Au
So;
84.9% / 15.1% = 5.6 g
There is 5.6 g of Au per gram of Cl
Also;
B contains 35.1% of Cl and 64.9% of Au
So;
64.9%/35.1% = 1.8 g
There is 1.8 g of Au per gram of Cl
Hence;
5.4/1.8, 1.8/1.8
3 : 1
Since the composition of the atoms in the compounds are in simple ratio to each other, they obey the law of multiple proportion.
Learn more about law of multiple proportion: https://brainly.com/question/25260214
which macro-molecule is considered an amino acid?
Answers
7. When solid carbon reacts with oxygen gas to produce carbon dioxide gas, woul the triangle H value be on the reactant or product side of the equation?
Answers
When solid carbon reacts with oxygen gas to produce carbon dioxide gas. the deltaH (enthalpy change ) value is negative .DeltaH would be on the product side of the equation.
What is enthalpy change?In a thermodynamic system, energy is measured by enthalpy. Enthalpy is a measure of a system's overall heat content and is equal to the system's internal energy plus the sum of its volume and pressure.
Knowing whether q is endothermic or exothermic allows one to characterise the relationship between q and H. An endothermic reaction is one that absorbs heat and demonstrates that heat from the environment is used in the reaction, hence q>0 (positive). For the aforementioned equation, under constant pressure and temperature, if q is positive, then H will also be positive. In a similar manner, heat is transferred to the environment when it is released during an exothermic reaction. Thus, q=0 (negative). Therefore, if q is negative, H will also be negative.
Learn more about enthalpy change here :
brainly.com/question/1445358
#SPJ13
1. You bought a loaf of bread and on the way home, the loaf of bread was crushed. Is the density of the crushed bread the same density as the uncrushed bread? If the density of the crushed bread is different from the density of the uncrushed bread, tell which bread has the higher density. Explain how you know.
Answers
Answer:
No. It is more dense than the uncrushed bread.
Explanation:
The mass is the same, however the volume has been decreased on the crushed side, forcing more matter into one space, which increases the density.
The loaf of bread was crushed then the density of crushed bread and uncrushed bread is same.
What is density?Density is defined as a measurement of how tightly the material is packed together.
It can also be defined as mass of a unit volume of a material substance.
Density can be expressed as
Density = mass / volume
The density depends on mass and volume and mass and volume of crushed and uncrushed bread is same only surface area changes with structural deformation.
Thus, the loaf of bread was crushed then the density of crushed bread and uncrushed bread is same.
To learn more about density, refer to the link below:
https://brainly.com/question/15164682
#SPJ2
How does Kinetic energy and how does it relates to diffusion and temperature.
HURRY HELPPP!!!!!!!!!!!!!!!!!!!!
Answers
Answer:
ok
Explanation:
khfdnkv Klein pick pick or en la Web outta
50 POINTS!!!! someone please quickly help and show work
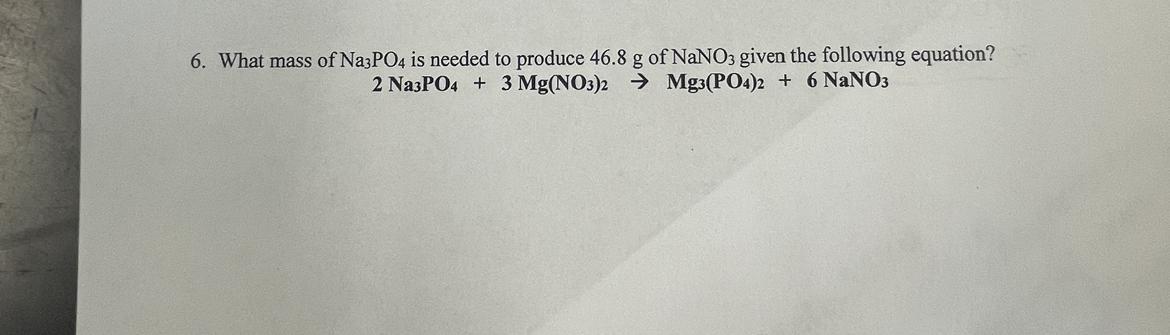
Answers
The mass of Na₃PO₄ needed to produce 46.8 grams of NaNO₃ in the given chemical reaction is 31.3 grams
How do i determine the mass of oxygen required?The mass of Na₃PO₄ needed to produce 46.8 grams of NaNO₃ can be obtain as follow:
2Na₃PO₄ + 3Mg(NO₃)₂ -> Mg₃(PO₄)₂ + 6NaNO₃
Molar mass of Na₃PO₄ = 164 g/molMass of Na₃PO₄ from the balanced equation = 2 × 164 = 328 g Molar mass of NaNO₃ = 85 g/molMass of NaNO₃ from the balanced equation = 6 × 85 = 510 gFrom the balanced equation above,
510 g of NaNO₃ were obtained from 328 g of Na₃PO₄
Therefore,
46.8 g of NaNO₃ will be obtain from = (46.8 × 328) / 510 = 31.3 g of Na₃PO₄
Thus, the mass of Na₃PO₄ needed to produced 46.8 g of NaNO₃ is 31.3 g
Learn more about mass needed:
https://brainly.com/question/29263739
#SPJ1
As we will soon see in Section 4.5, gasoline is a complex mixture of alkanes and other hydrocarbons, and the term "octane rating" is used as a standard measure of the performance of engine fuel. In the petroleum industry, one area of interest is the use of catalysts to convert unbranched alkanes and branched alkanes with higher octane ratings (Journal of the Japan Petroleum Institute 2004, 47, 1–10). For example, heptane can undergo isomerization in the presence of suitable catalysts to give eight different constitutional isomers, all of which are branched alkanes with the molecular formula C7H16. Draw all eight constitutional isomers, making sure not to draw the same compound twice.
Answers
Answer:
Explanation:
C₇H₁₆ is heptane and it has nine isomers (n-heptane and the remaining 8 constitutional isomers). Constitutional isomers are compounds with the same molecular formula but different structural formula or atom connectivity.
The constitutional isomers of n-pentane are 2-methylhexane, 3-methylhexane, 2,2-dimethylpentane, 2,3-dimethylpentane, 2,4-dimethylpentane, 3,3-dimethylpentane, 3-ethylpentane and 2,2,3-trimethylbutane. There structures are in the picture attached

Which has a higher lattice energy? Na3n or Na2O. And why?
Answers
Because the extent of ionic charge is more important than ionic radius in calculating the lattice energy of ionic compounds. Because the ionic charge of the N3-ion is greater than that of the F-ion, the Na3N molecule has a higher lattice energy than the NaF complex.
The lattice energy increases when the ion charge variable is increased. As a result, ions with higher charge values generate ionic compounds with higher lattice energies. As a result, ions with lower charges lower the lattice energies of their compounds.
help please it’s due soon just calculate the atomic mass of lithium that’s all i need

Answers
Which reaction will most likely take place based on the activity series?
Li > K > Ba > Ca > Na > Mn > Zn > Cr > Fe > Cd > Ni > H > Sb > Cu > Ag > Pd > Hg > Pt
a. Pt + FeCl3 Right arrow.
b. Mn + CaO Right arrow.
c. Li + ZnCO3 Right arrow.
d. Cu + 2KNO3 Right arrow.
Answers
Answer:
C
Explanation:
According to the activity series, Li can displace Zn to give LiCO3
Calculate the mass of vanadium(V) oxide (V2O5) that contains a billion (1.000e9) vanadium atoms. Be sure your answer has a unit symbol if necessary, and round it to 4 significant digits._______
Answers
Answer:
1.510 × 10⁻¹³ g
Explanation:
Step 1: Given data
Number of atoms of vanadium: 1.000 × 10⁹ atoms
Step 2: Calculate the molecules of V₂O₅ that contain 1.000 × 10⁹ atoms of V
Each molecule of V₂O₅ has 2 atoms of V.
1.000 × 10⁹ atom V × (1 molecule V₂O₅/2 atom V) = 0.5000 × 10⁹ molecule V₂O₅
Step 3: Calculate the moles corresponding to 0.5000 × 10⁹ molecules of V₂O₅
We will use Avogadro's number: there are 6.022 × 10²³ molecules of V₂O₅ in 1 mole of V₂O₅.
0.5000 × 10⁹ molecule × (1 mol/6.022 × 10²³ molecule) = 8.303 × 10⁻¹⁶ mol
Step 4: Calculate the mass corresponding to 8.303 × 10⁻¹⁶ moles of V₂O₅
The molar mass of V₂O₅ is 181.88 g/mol.
8.303 × 10⁻¹⁶ mol × 181.88 g/mol = 1.510 × 10⁻¹³ g
A spatula of sodium hydrogen carbonate was placed in a boiling tube.lemon juice was added dropwise while shaking until no other change was seen. Give the expected observation and explain it
Answers
Answer:
When sodium hydrogen carbonate (also known as baking soda) reacts with lemon juice (which is acidic), a chemical reaction occurs. The expected observations and the explanation for each observation are as follows:
1. Effervescence (bubbling): As the lemon juice (citric acid) reacts with sodium hydrogen carbonate, carbon dioxide gas is produced. This gas escapes as bubbles, leading to effervescence. The reaction can be represented as follows:
Sodium Hydrogen Carbonate + Citric Acid → Carbon Dioxide + Water + Sodium Citrate
2. Release of a citric-like odor: When citric acid from the lemon juice reacts with the sodium hydrogen carbonate, it forms sodium citrate, which has a fruity odor similar to citric acid.
3. Change in color or formation of foam: Depending on the specific lemon juice used, there might be a color change or the formation of foam due to the interaction between the citric acid and the baking soda. This observation can vary depending on the concentration of the lemon juice and the amount of baking soda used.
4. No further visible change: Once the reaction is complete, there will be no other visible changes. The carbon dioxide gas produced during the reaction will dissipate into the air, and the solution will reach a new equilibrium.
Overall, the reaction between sodium hydrogen carbonate and lemon juice is an acid-base reaction, resulting in the production of carbon dioxide gas. This reaction is commonly used in baking to create a leavening effect and make baked goods rise.
Discuss the following statement:
"Small changes in the chemical nature of polysaccharides results in significant differences in biological function"
Answers
Answer:
Explanation:
Small changes in the chemical nature of polysaccharides can make a big difference in how they work in our bodies. Polysaccharides are complex carbohydrates found in things like fiber and medicines. Even tiny changes in their structure can affect how they are digested, how they interact with cells, and their overall impact on our health. Scientists can use these changes to create materials with specific properties or develop new treatments. So, even small tweaks in polysaccharides can have a significant impact on how they function in our bodies.
Why do astronauts with less on the moon than they do on Earth
Answers
What is an alloy?
A. A metal with a changed oxidation state
B.a combination of a metal with another element
C.a layering of one metal over another metal
D. A substitution of one metal for another metal
Answers
Which reaction product (A or B) is more likely to form in the epoxide ring opening reaction? Consider resonance structures.
Answers
Answer:
Reaction product A is more likely to form in the epoxide ring-opening reaction
Explanation:
The diagram for the reaction can be seen in the image attached below.
An epoxide is any class of organic compound, cyclic ethers, having a three-membered ring; they are usually prepared by selective oxidation of alkenes or by ring-closure of halohydrins(any class of organic compound having a hydroxyl functional group and a halogen on neighboring carbon atom ) which are used in making plastic.

starting with toluene which sequence of reactions below works best to prepare the folowing cyclohexadiene compound
Answers
The toluene which is sequence of reactions below the works best to prepare the following cyclohexadiene compound is given as follows :
NBS , heat NaOCH₃ Na, NH₃
Toluene ------------------> -----------------> ------------>
CCl₄ CH₃OH CH₃OH
The toluene is the substituted aromatic hydrocarbon. the cyclohexadiene is used as the starting material for the synthesis of the natural complex products. the cyclohexadiene can be formed by the toluene . the synthesis which is the best to prepare the cyclohexadiene from the compound that is toluene is given as follows :
NBS , heat NaOCH₃ Na, NH₃
Toluene ------------------> -----------------> ------------>
CCl₄ CH₃OH CH₃OH
To learn more about toluene here
https://brainly.com/question/16243535
#SPJ4
PLEASE ANSWER SOON AS POSSIBLE
What is the volume, in liters, of 1.20 mol of C3H8 gas at STP
Answers
26.8L is the volume, in liters, of 1.20 mol of C\(_3\)H\(_8\) gas at STP. A measurement of three-dimensional space is volume.
A measurement of three-dimensional space is volume. It is frequently expressed quantitatively using SI-derived units, like the cubic metre or litre, or different imperial or US-standard units, including the gallon, quart and cubic inch. Volume and length (cubed) have a symbiotic relationship.
The volume of an envelope is typically thought of as its capacity, not as the amount of space it takes up. In other words, the volume is the quantity of fluid (liquid or gas) that the container may hold.
Volume = 1.20×22.4
=26.8L
To know more about volume, here:
https://brainly.com/question/1578538
#SPJ1
What might happen if water molecules did not have a slight negative charge on one end and a slight positive charge on another
Answers
Water molecules did not have a slight negative charge on one end and a slight positive charge on another, the loss of polarity would have profound effects on various biological, chemical, and physical processes. The unique properties of water that are vital for life as we know it would be significantly altered, potentially rendering many biological systems nonfunctional and disrupting the stability of ecosystems.
Loss of hydrogen bonding: The polarity of water molecules allows them to form hydrogen bonds with each other and with other polar substances.Hydrogen bonds are relatively weak but essential for various biological processes, including protein folding, DNA structure, and the stabilization of cell membranes. Altered solubility: Water's polarity contributes to its excellent solvent properties. It can dissolve a wide range of substances, including salts, sugars, and polar molecules, due to its ability to surround and separate charged or polar particles. Changes in boiling and freezing points: The polarity of water affects its boiling and freezing points. Water has a relatively high boiling point and melting point compared to other substances of similar molecular weight. Altered surface tension: Surface tension is the cohesive force that holds the surface of a liquid together. Water exhibits relatively high surface tension due to the cohesive forces between water molecules resulting from their polarity. Changes in heat capacity: Water's ability to absorb and retain heat is crucial for temperature regulation in many organisms and helps moderate temperature changes in the environment.For such more question on Water molecules
https://brainly.com/question/21426318
#SPJ8
ORDS ONLY. 71.A broad, painless, pink-gray, wart like infectious lesions may develop on the vulva, perineum, or anus in syphilis is called----- 72. In suspected syphilis infection the term RPR stands for? 73. The recommended dosage of Benzathine Penicillin in an adult in Zambia is je 74. The recommended drug for treatment of gonorrhea when using syndromic management is 75. The causative organism for chancroid is called- 76. The commonest type of HIV is 77.The assertive, problem solving approach to identification and treatment of the patient's problems is called --- 78. A tumour arising from the cells producing melanin is also known as 1 79. The type of sound described as drum like, loud, empty quality felt over gas-filled stomach, intestine or pneumothorax which is heard during percussion is called host wall into the pleural space to obtain
Answers
71. The broad, painless, pink-gray, wart-like infectious lesions that may develop on the vulva, perineum, or anus in syphilis are called "condyloma lata."
72. In suspected syphilis infection, the term RPR stands for "Rapid Plasma Reagin." It is a blood test used to screen for syphilis.
73. The recommended dosage of Benzathine Penicillin in an adult in Zambia may vary depending on the stage and severity of the syphilis infection. It is best to consult local guidelines or a healthcare professional for the specific recommended dosage in Zambia.
74. The recommended drug for the treatment of gonorrhea when using syndromic management may vary depending on local guidelines and antibiotic resistance patterns. Commonly used antibiotics include ceftriaxone in combination with azithromycin or doxycycline.
75. The causative organism for chancroid is called "Haemophilus ducreyi."
76. The commonest type of HIV is "HIV-1."
77. The assertive, problem-solving approach to the identification and treatment of the patient's problems is called "clinical decision-making."
78. A tumor arising from the cells producing melanin is also known as "melanoma."
79. The type of sound described as drum-like, loud, and empty quality felt over a gas-filled stomach, intestine, or pneumothorax during percussion is called "tympany."
To know more about melanoma:
https://brainly.com/question/14972277
#SPJ1
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
There is a homogeneous mixture of 3 solids: Sand, salt, and candle wax.
What could I do to easily remove the salt?
Answers
Answer:
Because mass is neither created nor destroyed, so the wax can not just disappear.
Explanation: