5. The atomic number of an element is equal to:
a. the number of protons in the atom
b. the number of protons plus the number of neutrons
c. the number of neutrons in the atom
d. the number of protons plus the number of electrons
Answers
Related Questions
1. A newspaper article wrote about a study in which researchers subjected laboratory gloves to stress. Among 240 vinyl gloves, 63% leaked. Among 240 latex gloves, 7% leaked. Calculate the claim that vinyl gloves have a higher leak rate than latex gloves. Use 0.005 significance level.
Answers
The claim that vinyl gloves have a higher leak rate than latex gloves is supported by the study at a significance level of 0.005.
To determine if vinyl gloves have a higher leak rate than latex gloves, we can conduct a hypothesis test.
The z-value is calculated as:
z = (p₁ - p₂) / √((p₁(1 - p₁) / n₁) + (p₂(1 - p₂) / n₂))
where p₁ and p₂ are the sample proportions, and n₁ and n₂ are the sample sizes.
Certainly! Let's calculate the z-value to determine if vinyl gloves have a higher leak rate than latex gloves.
For vinyl gloves:
Sample size (n₁) = 240
Leaking gloves (x₁) = 0.63 * 240 = 151.2 (approximated to 151)
For latex gloves:
Sample size (n₂) = 240
Leaking gloves (x₂) = 0.07 * 240 = 16.8 (approximated to 17)
We will calculate the z-value using the formula:
z = (p₁ - p₂) / √((p₁(1 - p₁) / n₁) + (p₂(1 - p₂) / n₂))
where p₁ and p₂ are the sample proportions.
p₁ = x₁ / n₁ = 151 / 240 ≈ 0.629
p₂ = x₂ / n₂ = 17 / 240 ≈ 0.071
Calculating the z-value:
z = (0.629 - 0.071) / √((0.629 * (1 - 0.629) / 240) + (0.071 * (1 - 0.071) / 240))
z ≈ 13.239
The calculated z-value is approximately 13.239. To determine if the claim is supported, we compare this value to the critical z-value for a one-tailed test at a significance level of 0.005.
learn more about significance level Here:
https://brainly.com/question/30766149
#SPJ4
A solid substance turns directly into a gas. Which term describes this change?
A)deposition
B)evaporation
C)melting
D)sublimation
Answers
Given what we know, we can confirm that the correct term to describe the change from a solid into a gas is sublimation.
What is sublimation?Sublimation is the change from a solid-state into a gas state.Most of the time one will first observe the change into a liquid state, however, with sublimation, the change is directly from the solid into the gas, such is the case with dry ice. Dry Ice is frozen carbon dioxide, as it thaws, it changes directly into its gaseous state.Therefore, we can confirm that sublimation describes the process by which an element passes from a solid directly into a gas, without first becoming a liquid.
To learn more about sublimation visit:
https://brainly.com/question/10936758?referrer=searchResults
Answer:
D. sublimation
Explanation:
Correct on Edge 2022!!!
Good luck everyone, you got this! Have a great day!
Why are you able to smell things across a room or form great distances
Answers
Answer:
We smell hot food from distance because of the diffusion process. Diffusion is the spreading out and intermixing of particles From one substance into another substance due to the movement of particles.
Explanation:
If one electron in an atom is excited but does not have enough energy to escape, the energy of the excited electron must have one of a set of quantized values.a. Trueb. False
Answers
help does anyone know this

Answers
| Adipic acid contains 49.32% C, 43.84% O, and 6.85% H by mass. What is the empirical formula? A) C3H5O₂ B) C3H3O4 C) C₂HO3 D) C₂H5O4
Answers
The empirical formula will be C₃H₃O₄
The smallest thought the entire ratio of the numerous atoms within a compound is represented by an empirical formula. The precise number of various atoms popular conceptions in a compound's molecule is indicated by the molecular formula.
It can be written as:
C= 49.32 g x (1 mol / 12.011) = 4.11 mol C
O= 43.84 g x (1 mol / 16.00) = 2.74 mol O
H= 6.85 g x (1 mol / 1.008) = 6.80 mol H
4.11 mol C / 2.74 = 1.5
2.74 mol O / 2.74 = 1
6.80 mol H / 2.74 = 2.5
It need a whole number so you find the least common multiple to make each one a whole number.
1.5 x 2 = 3 C
1 x 2 = 2 O
2.5 x 2 = 5 H
C₃H₃O₄
Therefore, the empirical formula is C3H3O4
To know more about Empirical Formula do visit
https://brainly.com/question/14044066
#SPJ1
which type of substance ionizes completely and creates hydronium ions when dissolved in water? (5 points) strong acid strong base weak acid weak base
Answers
The type of substance that ionizes completely and creates hydronium ions when dissolved in water is a strong acid.
When a substance is classified as a strong acid, it means that it ionizes completely when dissolved in water. In other words, all of the acid molecules dissociate into ions. Specifically, strong acids readily donate protons (H⁺ ions) to the surrounding water molecules, leading to the formation of hydronium ions (H₃O⁺). The H⁺ ions released by the acid combine with water molecules to form H₃O⁺.
The process of ionization can be represented by a chemical equation. For example, let's take hydrochloric acid (HCl) as an example of a strong acid:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
In this equation, HCl dissociates completely into H⁺ and Cl⁻ ions when it is dissolved in water. The H⁺ ions combine with water molecules to form H₃O⁺, which is responsible for the acidic properties of the solution.
It's important to note that strong acids are characterized by their ability to fully ionize in water, resulting in a high concentration of hydronium ions. This high concentration of hydronium ions contributes to the acidic nature of the solution and its ability to readily donate protons in chemical reactions.
On the other hand, weak acids do not completely ionize in water and exist in a state of equilibrium between the undissociated acid molecules and the ions. Weak acids partially dissociate, resulting in a lower concentration of hydronium ions compared to strong acids.
In summary, strong acids ionize completely when dissolved in water, generating hydronium ions (H₃O⁺) and contributing to the acidic nature of the solution.
Learn more about strong acid from the link given below.
https://brainly.com/question/29769012
#SPJ4
A 21.496 grams sample of magnesium is burned in air to form magnesium oxide and magnesium nitride. When the products are treated with water,2.813 grams of gaseous ammonia are generated. Calculate the amounts of magnesium nitride and magnesium oxide formed?
Answers
25.898 g is the amount of magnesium nitride and magnesium oxide formed.
Step1 2Mg(s)+ O2(g)-----> 2 MgO(s)
3Mg(s)+N2(g)------> Mg3N2(s)
Mg3N2(s)+ 6 H2O(l)---> 3Mg(OH)2 + 2 NH3(g)
Step2 Moles of NH3 = (2.813/17)
Moles of Mg3N2= (1/2) Moles of NH3 = (2.813/2x17)= 2.813/34
Mass of Mg3N2 = (2.813/34) x100 =8.274g (Molar mass of Mg3N2=100)
Step3 Mass of Mg in Mg3N2 =(72/100) x8.274 =5.957g
Mass of Mg converted in MgO= 21.496-5.957=15.539
Moles of MgO= Moles of Mg = 15.539/24
Mass of MgO = 15.539x40/24 =25.898 g.
Magnesium is a cofactor for over 300 enzymatic systems that regulate various biochemical reactions in the body, including protein synthesis, muscle and nerve function, glycemic control, and blood pressure regulation [1-3].
Learn more about magnesium at
https://brainly.com/question/5759562
#SPJ1
an object displaces 32 mL of water and a known density of 0.0625 g/mL. What is the mass of the object?
Answers
\(\\ \sf\longmapsto Density=\dfrac{Mass}{Volume}\)
\(\\ \sf\longmapsto Mass=Density\times Volume \)
\(\\ \sf\longmapsto Mass=0.0625(32)\)
\(\\ \sf\longmapsto Mass=2g\)
What geographic obstacle causes water to condense in an air mass as the air mass moves? Explain your answer.
A. Forests
B.oceans
C.prairies
D.mountains
Answers
Answer:
Mountains
Explanation:
Mountains are colder than the lower elevations. When humid air enters a cooler region, the saturated air begins to cool and this reduces the ability of the air to maintain the evaporated water is the gas state. Molecules of water have reduced enerfy at the cooler temperatures. They will slow down and coalesce with other water molecules to form small droplets of water. The atmoshere cannot hold these heaevier droplets and they begin to fall (i.e., rain).
One migh ask why does the air temperature drop at higher altitudes? Heat rises, so why shouldn't it be warmer on top a mountain, compared to the valley. The reason is that the atmosphere becomes less dense the further from Earth's center on gets. This less dense air has a lower water saturation point, so it rains.
What if a small amount of air leaked back into the flask through the tightened screw clamp as the flask assembly was cooling? would your calculated value for the molar mass of air be too high, too low, or would there be no effect? explain
Answers
The measured volume of hydrogen gas will be too high.
The volume of hydrogen is measured by collecting the gas over water. The volume of the gas is measured as the volume of water displaced by the gas in an inverted container.
When air leaks into the graduated cylinder, more volume of water is displaced hence a higher volume of hydrogen gas is measured.The calculated molar volume of hydrogen will be too high as a result of this error
The gas being recorded during the experiment would not only include hydrogen gas, but the air that leaked into the eudiometer tube as well. This leads to the increase in the volume of hydrogen gas which will be too high.
Since the volume of hydrogen is too high, therefore the calculated molar volume of hydrogen would also be too high.
To know more about volume, refer: brainly.com/question/10609459
#SPJ4
help please
Analyze the graph to compare the energy and greenhouse emissions generated by different sources of energy. Based on the graph, would you support nuclear energy?

Answers
Answer:
Yes
Explanation:
Based on the graph, nuclear energy is one of the least contributors of CO2 emissions.
You would support this source of energy .
An increase in the bat population would most likely result in
А
Decrease in mold growth
B
An increase in the outisde insect population
с
A decrease in bat bug population
D
A decrease in the outside insect population
Answers
Answer:
Explanation:
D
A decrease in the outside insect population
A science student wants to use distillation to separate a mixture of two substances in the liquid state. For the student to be successful, which property should be significantly different between the two liquids?
Answers
For a science student to be successful in separating a mixture of two substances in the liquid state using distillation, the boiling points of the two liquids should be significantly different.
What happens during a distillation?In distillation, a mixture of liquids is heated, and the vapor produced is condensed and collected. The liquids in the mixture will have different boiling points, so the substance with the lower boiling point will vaporize and condense at a lower temperature compared to the substance with the higher boiling point.
What is necessary for a successful distillation?For a successful distillation, it is important that the boiling points of the two liquids are significantly different so that they can be separated easily. If the boiling points of the two liquids are too close, it will be difficult to separate them using distillation.
To know more about boiling point, visit here:
https://brainly.com/question/2153588
#SPJ1
acids are and can destroy your skin and bones
Answers
Answer:
This is true.
Explanation:
Help me please What makes water filters better
Answers
Answer:
well according to salesman they take out all and out all bacteria that is in the water that the water filters installed in the systems already don't take out so in other wordsthere and the water filter takes that out
what material should a coffee mug be constructed from?
A. paper.
B. metal.
C. clay.
D. stone
Answers
Answer:
The answer is Clay or C!!!
Explanation:
Brainliest Please!!!!!!!!!
6v
Next
Pretest: Chemical Quantities
Gas Laws Fact Sheet
Ideal gas law
Ideal gas constant
Standard atmospheric pressure
Celsius to Kelvin conversion
16
PV = nRT
R= 8.314
or
The water bottle contains
LkPa
mol K
Type the correct answer in the box. Express your answer to three significant figures.
An empty water bottle is
mole of air.
R=0.0821 Lam
1 atm = 101.3 kPa
K="C + 273.15
full of air at 15°C and standard pressure. The volume of the bot0.500 liter. How many moles of air are in the bottle?
Reset
Submit Test
Next
Reader Tools
Answers
0.0213 moles of air in the water bottle at 15°C and standard pressure.
To determine the number of moles of air in the water bottle, we can use the ideal gas law equation:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature.
In this case, we are given the volume of the bottle (V = 0.500 liters), the temperature (T = 15°C = 15 + 273.15 = 288.15 K), and the pressure (standard pressure = 1 atm = 101.3 kPa).
First, we need to convert the pressure to atm. Since 1 atm = 101.3 kPa, the pressure in atm is 1 atm.
Now, we can rearrange the ideal gas law equation to solve for the number of moles:
n = PV / RT
Substituting the given values and the value of the ideal gas constant (R = 0.0821 L·atm/(mol·K)), we can calculate the number of moles of air:
n = (1 atm) × (0.500 L) / (0.0821 L·atm/(mol·K) × 288.15 K)
After performing the calculations, we find that the number of moles of air in the water bottle is approximately 0.0213 moles.
Know more about ideal gas law here:
https://brainly.com/question/27870704
#SPJ8
The initial molar concentration of the inside of a cell is 2M and the cell is placed in a solution with a concentration of 2.5M. Assuming that the membrane is not permeable to the solute, answer the following questions with a T or F. If the answer is false, re-write it to be a true statement. (1 mark each) a) Initially, the cytoplasm is hypertonic to the surrounding solution.| b) Net diffusion of water will be from inside the cell to outside the cell. c) After movement of materials, the molarity of the cytoplasm will have increased. d) If the membrane was permeable to the solute, water would still move in the same direction.
Answers
The initial molar concentration of the inside of a cell is 2M and the cell is placed in a solution with a concentration of 2.5M. Assuming that the membrane is not permeable to the solute, the answer to the following questions is given below:
a) Initially, the cytoplasm is hypotonic to the surrounding solution because the solute concentration outside the cell is more than the solute concentration inside the cell, so water will move from inside the cell to outside the cell. Hence, the cytoplasm is hypotonic to the surrounding solution.
b) Net diffusion of water will be from inside the cell to outside the cell. As the surrounding solution is hypertonic and the cytoplasm is hypotonic, water moves outside of the cell, making this statement true.
c) After the movement of materials, the molarity of the cytoplasm will have increased. After the movement of materials, the molarity of the cytoplasm will have decreased because the water will move out of the cell.
d) If the membrane was permeable to the solute, water would still move in the same direction. Whether the membrane is permeable to the solute or not, the direction of water movement remains the same.
Hence, this statement is true.
To know more about molar concentration, visit:
https://brainly.com/question/21841645
#SPJ11
Identify the rule, if any, being violated in this example:
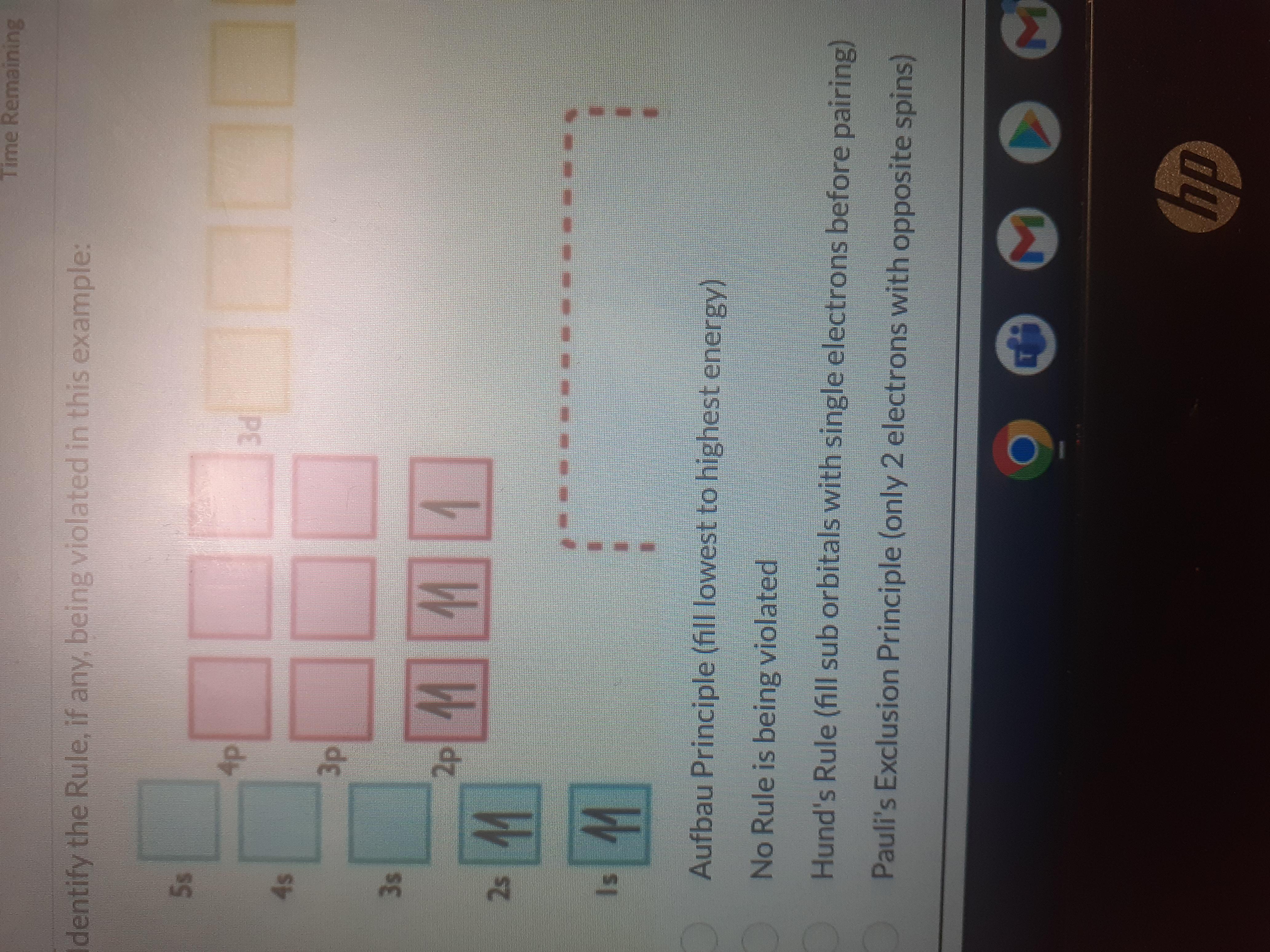
Answers
In the given example, Pauli's exclusion principle is violated. Therefore, option (D) is correct.
What is Pauli's exclusion principle?The Pauli exclusion principle can be described as no two electrons will have similar four quantum numbers (n, l, ml, and ms) in an atom. Every electron should have a unique state (singlet state) or set of quantum numbers.
According to this principle, only two electrons can fill the same orbital. The two electrons in the same orbital must have opposite or antiparallel spins.
If an orbital has only one electron then it can have spin-up or spin-down. If there are two electrons in an orbital, then one electron must have a spin-up and the other should have a spin-down state but not the same.
Therefore, the two electrons in the same orbital can never have the same spins.
Learn more about Pauli's exclusion principle, here:
brainly.com/question/3454051
#SPJ1
the properties of a compound are ____ the properties of the element that form it
Answers
What is the volume of 5 moles of oxygen gas at r.t.p ?
Answers
Explanation:
\(5mol \times \frac{22.4lit}{1mol} = 112lit\)
If you start with 52 kg of a radioactive isotope, calculate how much of the original isotope is left after two half-lives have elapsed. A. How many kg of the isotope remains? B. What fraction of the isotope remains?
Answers
If you start with 52 kg of a radioactive isotope, 13 kg of the isotope remains. The fraction of the isotope remains is 25%.
Radioactive isotopes decay over time, and the amount of the original isotope remaining can be calculated using the isotope's half-life. The half-life of an isotope is the amount of time it takes for half of the atoms in a sample to decay. The half-life of the isotope in question is unknown, so it cannot be determined.
However, we can use the half-life to calculate the amount of the original isotope remaining after a given period of time. After one half-life, half of the original isotope will have decayed. So after two half-lives, only a quarter of the original isotope will remain.
A) If we start with 52 kg of a radioactive isotope, and only a quarter of it remains after two half-lives have elapsed, then the amount remaining can be calculated as follows:
52 kg × 0.25 = 13 kg
Therefore, 13 kg of the isotope remains.
B) To calculate the fraction of the isotope remaining, we need to divide the amount remaining by the original amount:
Fraction remaining = 13 kg / 52 kg
= 0.25
Therefore, 0.25 or 25% of the isotope remains.
Learn more about radioactive isotope-
brainly.com/question/28039996
#SPJ11
why is an acetyl group added to aniline (making acetanilide) and then re- moved to regenerate the amine group in sulfanilamide?
Answers
An acetyl group is added to aniline to increase its solubility and stability, and then it is removed to restore the amine group in sulfanilamide.
In what ways does an acetyl group enhance stability?The acetyl group (CH3CO-) contains a carbonyl group (C=O) bonded to a methyl group (CH3). This arrangement allows for resonance stabilization, where the π-electrons of the carbonyl group can delocalize and spread out over the adjacent atoms. Resonance contributes to stability by distributing the electron density and reducing localized charge buildup.
Additionally, the carbonyl group has electron-withdrawing properties. The oxygen atom in the carbonyl group is more electronegative than carbon, creating a polar bond. This polarization withdraws electron density from the carbon atom, making it less susceptible to nucleophilic attacks and oxidation.
The combination of these both effects makes the acetyl group more stable compared to a simple alkyl group.
The subsequent removal of the acetyl group is achieved by a process called hydrolysis. By treating acetanilide with an appropriate reagent, such as an acid or a base, the acetyl group is cleaved, regenerating the original amine group in sulfanilamide. This conversion is important because the amine group is often the reactive site in various biological and chemical processes.
Learn more about:Resonance stabilization
brainly.com/question/30490931
#SPJ11
explain why it is necessary to change models as new information becomes available.
Answers
Answer:
Scientific models are not a replacement for experimentation. They can be used in conjunction with experimentation to further understanding of a concept, event, or process. They are based on current scientific knowledge and may have to be changed when new discoveries are made.
Explanation:
that's my answer
#carryonlearning
A rigid, 2. 50 L bottle contains 0. 458 mol He. The pressure of the gas inside the bottle is 1. 83 atm. If 0. 713 mol Ar is added to the bottle and the pressure increases to 2. 05 atm, what is the change in temperature of the gas mixture? Use the correct number of significant digits. Formula: PV = nRT(R = 0. 0821 Les001-1. Jpgatm/moles001-2. JpgK) The initial temperature of the gas is K.
Answers
The change in temperature with the addition of Ar to He gas is 68.61 K. The negative change indicates the lowering of the temperature of gas.
What is an ideal gas equation?The pressure of an ideal gas is given by the ideal gas equation as:
PV=nRT
The initial temperature of He gas is given as:
Initial pressure, P=1.83 atmThe initial volume, V=2.50 LInitial moles of gas, n=0.458 molThe value of the gas constant, R=0.0821 J/mol.KSubstituting the values for initial temperature, \(T_i\):
\(T_i=\rm \dfrac{1.83\;atm\;\times\;2.50\;L}{0.458\;mol\;\times\;0.082\;J/mol.K} \\\textit T_\textit i=122\;K\)
The initial temperature of the gas is 122 K.
The addition of 0.713 mol Ar, results in the final values of:
Final pressure, P=2.05 atmFinal volume, V = 2.50 LFinal moles, n=1.171 molSubstituting the values for final temperature, \(T_f\):
\(T_f=\rm \dfrac{2.05\;atm\;\times\;2.50\;L}{1.171\;mol\;\times\;0.082\;J/mol.K} \\\textit T_\textit f=53.3\;K\)
The final temperature of the gas is 53.3 K.
The change in temperature (\(\Delta T\)) is given as:
\(\Delta T=T_f-T_i\\\Delta T=53.3-122\;\text K\\\Delta T=-68.61\;\text K\)
The change in temperature of the gas is 68.61 K. The negative sign indicates the lowering of temperature with the addition of Ar.
Learn more about ideal gas, here:
https://brainly.com/question/8711877
Answer:
answer in picture
Explanation:

Identify the type of wave that would be located at Point A below.

Answers
A student investigates the number of particles of water that exist in a closed test tube throughout the phase
change of liquid to gas.
How many particles will be in the test tube after the water vaporizes and turns into a gas?
Answers
The number of particles of water that exist in a closed test tube after the water vaporizes and turns into a gas will be the same as the number of particles before the phase change.
This is because during the phase change, the molecules of water simply change their state from liquid to gas.the phase change from liquid to gas does not involve any change in the number of molecules, only a change in the physical state of the molecules. The molecules do not disappear or gain additional molecules from outside the test tube. As such, the number of particles of water in the test tube after the phase change is the same as before the phase change.
learn more about phase change Refer:brainly.com/question/30270780
#SPJ1
part 2 out of 4 select all the suitable oxidizing agents for the previous reaction. h2 and a pt, pd, ni, or ru catalyst pcc in ch2cl2 lialh4 in diethyl ether, then h2o h2cro4 generated from na2cr2o7 in aqueous sulfuric acid nabh4 in ch3oh
Answers
Cl2 + 2NaBr 2NaCl + Br2 was the equation that is suitable for oxidizing agent.
what is an oxidizing agent ?
An oxidising agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance that obtains or "accepts"/"receives" an electron from a reducing agent in a redox chemical process (called the reductant, reducer, or electron donor). An oxidizer, in other terms, is any substance that oxidises another material. The oxidizer's oxidation state, which specifies the degree of electron loss, falls while the reductant's oxidation state increases; this is described by stating that oxidizers "undergo reduction" and "are reduced," whereas reducers "undergo oxidation" and "are oxidised." Oxygen, hydrogen peroxide, and halogens are common oxidising agents.
An oxidising agent is a chemical species that conducts a chemical reaction in which one or more electrons are gained.
Cl2 + 2NaBr 2NaCl + Br2 was the equation.
To learn more about oxidizing agent follow the given link: https://brainly.com/question/11387475
#SPJ4
Which noble gas will have the narrowest curve for distribution of molecular velocity?
Select the correct answer below:
He
Ne
Ar
Kr
Answers
The noble gas that will have the narrowest curve for the distribution of molecular velocity is helium (He).
The distribution of molecular velocity, also known as the Maxwell-Boltzmann distribution, describes the distribution of velocities for particles in a gas at a given temperature. The distribution curve is characterized by its width, which indicates the range of velocities of the gas molecules.
Helium (He) is the lightest noble gas with the lowest molecular mass. Due to its lower mass, helium molecules have higher average velocities compared to the other noble gases. As a result, the distribution curve for helium will be narrower, indicating a smaller range of velocities compared to the heavier noble gases.
In contrast, the heavier noble gases like Ne (neon), Ar (argon), and Kr (krypton) have higher molecular masses and, therefore, lower average velocities. Consequently, their distribution curves will be wider, indicating a broader range of velocities for the gas molecules.
To learn more about noble gas, click here: brainly.com/question/29000866
#SPJ11