After reduction with LiAlH4, followed by aqueous workup (protonation) the following starting materials will form what?
Carboxylic acids
Esters
Amides
Answers
After reduction with LiAlH4, followed by aqueous workup (protonation), carboxylic acids, esters, and amides will form their corresponding alcohols, primary alcohols for carboxylic acids and esters, and secondary alcohols for amides.
LiAlH4 is a strong reducing agent that is capable of reducing carbonyl functional groups, which include carboxylic acids, esters, and amides, to their corresponding alcohols. The aqueous workup (protonation) serves to neutralize the reaction mixture and remove any remaining LiAlH4. The resulting alcohols can then be further reacted or purified as desired.
After reduction with LiAlH4 (lithium aluminum hydride), followed by aqueous workup (protonation), the starting materials will undergo the following transformations:
1. Carboxylic acids: Reduction with LiAlH4 will convert carboxylic acids into primary alcohols.
2. Esters: LiAlH4 reduction will convert esters into a pair of alcohols, including a primary alcohol from the carbonyl group and an alcohol derived from the ester's alkyl/aryl group.
3. Amides: Reduction using LiAlH4 will transform amides into amines.
In summary, LiAlH4 is a strong reducing agent that can reduce carboxylic acids, esters, and amides to their corresponding alcohols or amines through the process of reduction followed by aqueous workup.
For more information on LiAlH4 visit:
brainly.com/question/30904954
#SPJ11
Related Questions
Adding nh3 (ammonia) to water makes nh4 . what will be the general ph of this new solution, and what will happen to the ion concentrations?
Answers
Adding NH3 to water makes NH4 . the pH of this new solution is alkaline. in the ion concentrations , there is a decreased H+ ions and increased OH-.
Ammonia is a compound of nitrogen and hydrogen . it is a stable binary hydride . it is a colorless gas with pungent smell . it is used as fertilizer , refrigerant gas , for purification of water , manufacture of plastic , pesticides , textile , dyes .
What is pH ?pH is the measure of acidity and basicity of a substance .
pH stands for hydrogen potential . it can be defined as the negative log of hydrogen ion concentration .
it can be measured by a pH scale , which ranges from 0 to 14 .
if the pH value is less than 7 , then the compound is called as acidic .
if the Ph value is greater than 7 , then the compound is called as basic .
if the pH is 7 , then the compound is called as neutral compound .
pH equals to 0 , called strongly acidic solution . pH equals to 14 is called strongly basic solution .
pH does not have units .
The formula for pH is given by ,
pH = - log[H+]
Limitations of pH scale :pH zero for 1N solution of strong acid .
pH is negative for concentrations 2N, 3N ,10N of strong acids .
Learn more about pH here :
brainly.com/question/26424076
#SPJ4
4.18 A material has dielectric constant 2.4 and loss-tangent 0.1 at 1 GHz. Find the complex propagation constant of a plane wave in the medium at 1 GHz and 10 GHz. 4.19 If the electric field inside the material in Problem 4.18 is 10 V/m at 6 GHz, find the intrinsic impedance of the medium and the complex magnetic field.
Answers
4.18At 1 GHz, the dielectric constant and loss tangent of a material are 2.4 and 0.1, respectively. Calculate the complex propagation constant of a plane wave in the medium at 1 GHz and 10 GHz.4.19.
4.18:Given that dielectric constant, εr = 2.4;Loss-tangent, tanδ = 0.1;Frequency, f = 1 GHz and 10 GHz;Formula used:C = c/ √ (εr-j tan δ)Where c is the speed of light;C is the complex propagation constant.At 1 GHz,C = 3 × 10^8/ √ (2.4-j 0.1)≈ 8.1241 + j0.3767Using the same formula at 10 GHz,C = 3 × 10^8/ √ (2.4-j 1)≈ 7.0701 + j3.2192Therefore, the complex propagation constant at 1 GHz and 10 GHz are 8.1241 + j0.3767 and 7.0701 + j3.2192, respectively.4.19
Given that;Electric field, E = 10 V/m;Frequency, f = 6 GHz.Intrinsic impedance of the medium, Z0 is given as: Z0 = (μ0/ε0)1/2 √ (1 + j tan δ/ εr)where εr is the dielectric constant;tan δ is the loss tangent;ε0 is the electric constant and μ0 is the magnetic constant.Substituting the given values, we have;Z0 = (4π × 10^-7 / 8.85 × 10^-12)1/2 √ (1 + j (0.1)/ 2.4)≈ 165.74 + j6.9568The magnetic field intensity can be calculated using the following formula:H = E/Z0 = (10/165.74 -j6.9568)≈ 0.0566 -j0.0075Therefore, the intrinsic impedance of the medium and the complex magnetic field are 165.74 + j6.9568 and 0.0566 -j0.0075, respectively.
To know more about dielectric visit:
https://brainly.com/question/31426793
#SPJ11
what happened when a small stream of cold water was run over the bottom of the florence flask? explain your observations by using the phase diagram of water.
Answers
When a small stream of cold water is run over the bottom of a Florence flask, the temperature of the flask decreases. As a result, the water vapor inside the flask condenses, turning from gas to liquid phase. This is observed as droplets forming on the inner surface of the flask.
Using the phase diagram of water, this phenomenon can be explained as follows:
1. Initially, the water vapor inside the flask is in the gaseous phase, as it's at a higher temperature and pressure compared to the cold water outside.
2. When the cold water stream contacts the flask, it causes the temperature of the flask's surface to decrease, which in turn lowers the temperature of the water vapor inside.
3. As the temperature of the vapor drops, it reaches the liquid-vapor equilibrium line on the phase diagram. This is the point where the vapor and liquid phases coexist at a specific temperature and pressure.
4. The water vapor then condenses into liquid droplets as it crosses the liquid-vapor equilibrium line, moving from the gaseous phase region to the liquid phase region on the phase diagram.
In summary, running a stream of cold water over the bottom of a Florence flask causes the water vapor inside to condense into liquid droplets, as explained by the phase diagram of water.
Learn more about flask here:
brainly.com/question/28389930
#SPJ11
What is the ratio of the number of molecules in 1 L of CO2(g) to the number of molecules in 1 L of CH4(g) at the same temperature and pressure? * 2 points 1:1 3:5 1:2 2:4
Answers
Answer:
1:1
Explanation:
Based on Avogadro's law, the volume of an ideal gas is directly proportional to the moles (Or number of molecules) presents of this gas under constant temperature.
The volume of the gas is independent to the nature of the gas
Using this law, we can change the state as:
X number of molecules of CO₂ in X number of molecules of CH₄ (Because are occupying the same volume). That means the ratio of molecules is:
1:1All the simple machines make work easier to do by changing the _____ or _____ of a force. A. size; type B. work; type C. size; direction D. type; direction
Answers
Answer:
C. size; direction
Explanation:
By definition, a machine is referred to any device that makes work easier. It takes force to do work, hence, work refers to the application of force over a particular distance. A machine aims at making the work easy by changing how it is done. Simple machines, which include: levers, pulleys, inclined planes etc. all carry out the same thing, which is to make work easier, by changing the size/magnitude and direction of the applied force.
A simple machine tends to change the size of the inputted force by increasing it over a shorter distance. The machine increases the force applied better than it can be done manually e.g. a plier and nutcracker increases/changes the applied force better than it can be done with bare hands.
Also, a simple machine can achieve making work easier by changing the direction at which the force is applied. The machine applies the force on the object in an opposite direction or contrary to the way it was manually applied.
Find the lattice energy of MgH2(), how with a erie of tep when the Enthalpie of formation for calcium hydride i given a; (ΔHf = −75. 3 kJ/mol for MgH2)
Answers
the lattice energy of MgH2() is 2791 kJ/mol with a series of step when the Enthalpies of formation for calcium hydrided is given.
Do energies journal free?An article publishing charge (APC) is required of authors or research funders to cover publication costs in open access journals that do not charge subscription fees. This guarantees that everyone will be able to access your article promptly and without charge in the future.
Solution:
the lattice energy of MgH2() is :
ΔHf(−75. 3 kJ/mol ) =S+0.5D+IE+EA+U
ΔHf(−75. 3 kJ/mol )= 2791 kJ/mol
How good are MDPI journals?The papers published in MDPI Special Issues are of extremely high quality! When I published my research with MDPI (Energies Journal), it was a wonderful experience. Both the submission of the manuscript and the timing of publishing were excellent.
To know more about energy visit
brainly.com/question/11399976
#SPJ4
How many formula units are in 1.50 moles of NaCl?
Answers
Answer:
0.017
Explanation:
he reacting substrate carbon atom in the mechanism described in the passage undergoes which of the following hybridization state changes during the reaction? (note: the middle hybridization state refers to an intermediate.)
Answers
sp2 --> sp3 --> sp2
Enzymes hydrolyze peptide bonds. It does this thru a substitution reaction where the water molecule (this becomes deprotonated by means of the E residue to form a hydroxide nucleophile) attacks the sp2 hybridized carbon atom of the peptide bond. The resulting structure is an sp3 hybridized intermediate; the double bond between the carbon and oxygen turns into a single bond, and the electrons are driven onto the oxygen atom giving it a poor fee. This is unstable and the double bond reforms quickly after, and the C-N bond breaks.
The electron is a subatomic particle with a bad standard electric charge. Electrons belong to the primary technology of the lepton particle's own family and are typically thought to be fundamental particles because they have no recognized additives or substructure
.
For most realistic purposes, an electron is a structureless particle with an intrinsic angular momentum or spin. simply two numbers — the electron's mass and its electric price — gasoline the equations that describe its behavior. From this 'sensible electron' version, physicists constructed present-day microelectronics.
By using the best values for the wave length and the scattering by a matter of tough X-rays and ?-rays, the radius of the electron is anticipated as approximately 2 × 10-10 cm.
Learn more about Electron here:-https://brainly.com/question/860094
#SPJ4
what does the name cambrian period mean?
Answers
Answer:
the first geological time period of the Paleozoic Era
Explanation:
Who am I? Periodic table 20 questions answers
Answers
the answers to the questions based on the element sodium:
Is it metal? - Yes
Is it a non-metal? - No
Is it gas at room temperature? - No
Is it a solid at room temperature? - Yes
Is it a liquid at room temperature? - No
Is it in the first row (period) of the periodic table? - No
Is it in the second row (period) of the periodic table? - Yes
Is it in the third row (period) of the periodic table? - No
Is it in the fourth row (period) of the periodic table? - No
Is it in the fifth row (period) of the periodic table? - No
Is it in the sixth row (period) of the periodic table? - No
Is it in the seventh row (period) of the periodic table? - No
Is it in the eighth row (period) of the periodic table? - No
Is it a noble gas? - No
Is it a halogen? - No
Is it an alkali metal? - Yes
Is it an alkaline earth metal? - No
Is it a transition metal? - No
Does its symbol start with the letter "C"? - No
Does it have an atomic number greater than 50? - No (Sodium has an atomic number of 11)
Periodic Table 20 Questions" is a game where one player thinks of an element from the periodic table, and the other player asks up to 20 yes or no questions to guess the element.
The questions are usually related to the element's properties, such as its atomic number, symbol, group, or period, as well as its physical and chemical characteristics.
learn more about the Periodic table here
https://brainly.com/question/15987580
#SPJ1
the question is incomplete. complete question is
The element is sodium(Na)
Is it metal? - Yes or No
Is it a non-metal? - Yes or No
Is it gas at room temperature? - Yes or No
Is it a solid at room temperature? - Yes or No
Is it a liquid at room temperature? - Yes or No
Is it in the first row (period) of the periodic table? - Yes or No
Is it in the second row (period) of the periodic table? - Yes or No
Is it in the third row (period) of the periodic table? - Yes or No
Is it in the fourth row (period) of the periodic table? - Yes or No
Is it in the fifth row (period) of the periodic table? - Yes or No
Is it in the sixth row (period) of the periodic table? - Yes or No
Is it in the seventh row (period) of the periodic table? - Yes or No
Is it in the eighth row (period) of the periodic table? - Yes or No
Is it a noble gas? - Yes or No
Is it a halogen? - Yes or No
Is it an alkali metal? - Yes or No
Is it an alkaline earth metal? - Yes or No
Is it a transition metal? - Yes or No
Does its symbol start with the letter "C"? - Yes or No
Does it have an atomic number greater than 50? - Yes or No
Please help with this:
Typically, hard water used in a lab class would have been prepared by adding 1 gram of
magnesium sulfate per liter of distilled water. Magnesium sulfate contains 20.2%
magnesium ions by mass. What is its hardness in grains per gallon (GPG)? (One GPG
equals 17.1 mg/L.)
Answers
One grain of calcium carbonate, or 64.8 milligrams, is dissolved in one US gallon of water to represent one grain per gallon (gpg), a measure of water hardness (3.785412 L).
What is Water Hardness Measurement Scales?Understanding your test findings necessitates familiarity with the many water hardness testing scales that are employed. The majority of results are provided as a number that indicates the amount of calcium carbonate or calcium carbonate equivalents present in a specific unit of water. Depending on the measurement method, this value may be given in grains per gallon (gpg), parts per million (ppm), or milligrammes per litre (mg/L).Per Gallon of Grains Measurement of water hardnessThe hardness scale, expressed in gpg of calcium carbonate, can be seen as follows, according to the Water Quality Association:Soft is defined as gpg less than 1.An intermediate level of difficulty is between 1 and 3.5 gpg.The category of fairly challenging ranges from 3.5 to 7 gpg.The hard range is between 7 and 10.5 gpg.It's regarded quite difficult to get above 10.5 gpg.To Learn more About grain per gallon refer to:
https://brainly.com/question/2350304
#SPJ1
how many moles of NaCl can be formed from 6 moles of Cl2?
Answers
Answer:
12 moles
Explanation:
Which crystal system(s) listed below has (have) the following interaxial angle relationship?
Triclinic
Tetragonal
Orthorhombic
Monoclinic
Hexagonal
Rhombohedral
Cubic
Answers
The crystal system that exhibits the specified interaxial angle relationship is the hexagonal system.
The crystal system that has the following interaxial angle relationship is the hexagonal system. In the hexagonal system, the interaxial angle between any two crystallographic axes is 120 degrees, which is a characteristic feature of this system. The other crystal systems, including triclinic, tetragonal, orthorhombic, monoclinic, rhombohedral, and cubic, do not have a fixed interaxial angle relationship. Each of these systems has its own unique set of interaxial angles that define their crystal structures. Therefore, the interaxial angle relationship specified in the question specifically corresponds to the hexagonal crystal system.
Know more about Crystals here:
https://brainly.com/question/1283210
#SPJ11
Dhow only the set-ups needed to solve the following problem. A final answer is not needed.
Help please

Answers
1. The equation is 2KCl + F2 → 2KF + Cl2
2. The moles is 0.4 moles
3. The mole ratio is 2:1
4. 0.2 moles of chlorine is produced
5. The volume of the chlorine gas is 4.48 L
What is the stoichiometry?We have that the reaction equation is;
The balanced reaction equation is;
2KCl + F2 → 2KF + Cl2
The moles of the KCl = 30 g/75 g/mol
= 0.4 moles
If 2 moles of KCl produced 1 mole of chlorine
0.4 moles of KCl will produce 0.4 * 1/2
= 0.2 moles
If 1 mole occupies 22.4 L
0.2 moles occupies 0.2 * 22.4/1
= 4.48 L
Learn more about stoichiometry:https://brainly.com/question/30808199
#SPJ1
which is the correct answer ?
Protein-, peptide-, and amine-based hormones have which of the following in common? amino acids gluccose O Lipids and steroids Cholesterol
Answers
Protein, peptide and amine-based hormones have amino acids in common. Amino acids are the building blocks of protein. The synthesis of these hormones takes place in the endocrine glands which are responsible for producing and releasing hormones into the bloodstream.
The amino acids which make up these hormones, are linked together to form a long chain of amino acids, which can be broken down into smaller peptides. These peptides are then modified in various ways to produce the final hormone. The modification can include the addition of carbohydrates, lipids, and other molecules.
The hormones are then transported through the bloodstream to their target cells where they bind to specific receptors on the surface of the cell. Once bound, the hormone can either activate or inhibit certain cellular functions which results in a specific physiological response.In conclusion, the correct answer is that protein-, peptide-, and amine-based hormones have amino acids in common.
To know more about hormones visit:-
https://brainly.com/question/30367679
#SPJ11
How does thermal energy flow between a hot or cold pack and the atmosphere?
Answers
Answer:
Heat is the transfer of energy. During energy transfer, the energy moves from the hotter object to the colder object. This means that the hotter object will cool down and the colder object will warm up. The energy transfer will continue until both objects are at the same temperature.
Explanation:
a 13 letter word for a region around the nucleus of an atom whose electrons are likely to be found?
Answers
electron shell
Explanation:
Jerome is learning how the model of the atom has changed over time as new evidence was gathered. He has images of four models of the atom, but they are not in the correct order. An image at the left labeled W with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them. An image at center-left labeled X with a purple ball in the center surrounded by overlapping concentric black ovals, each with a small green ball on each of the 6 ovals. An image at center right labeled Y with a large black cross in a purple circle with a black line around the purple, with 10 small green balls dispersed within the purple circle. An image at right labeled Z with a purple center outlined in black with two concentric black circles around the center, the inner circle having 2 small green balls on it and the outer circle having 8 small green balls on it. In what order should Jerome put these models to show the development from the earliest model of the atom to the most recent one? A. Y, X, Z, W B. Y, Z, W, X C. W, Z, X, Y D. W, Y, Z, X
Answers
Answer:
the answer is A. Y, X, Z, W
Explanation:
I took the test
Answer:
a, y, x, z, w
Explanation:
Explanation:
silver (I) nitrate reacts with nickel (II) chloride to produce silver (I) chloride and nickel (II) nitrate wright the balanced chemical equation for this
Answers
Answer: 2 AgNO3(aq) + NiCl2(aq) ⇒ 2 AgCl(s) + Ni(NO3)2 (aq)
Explanation:
For each of the formulas, classify the formula as either an empirical formula, a molecular formula, or both. Nahco3 c2h6.
Answers
Sodium bicaronate's empirical and molecular formulas are both NaHCO3. The empirical formula for C2 H6 is CH3.
What does "structural formula" mean?
Chemical bonds connecting the atoms of a molecule are located in structural formulae. A structural formula is made up of symbols for the atoms joined by brief lines that stand in for chemical bonds. Single, double, and triple bonds are denoted by one, two, or three lines, respectively.
If we look at the molecular formula for NaHCO3, we can see that it has a molar ratio of 1:1:1:3 and that everything in this molar ratio results in a total mass of: 23g + 1g + 12g + 48g = 84g. Knowing that the molecular formula shows the total molar ratio of each element in a compound, while the empirical formula shows the simplest ratio of the elements present in a compound.
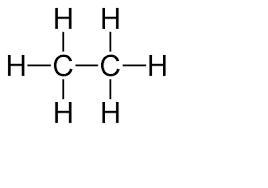
To determine the empirical formula of C2 H6 , you divide the molecular formula by a number that gives the smallest ratio of atoms. So, we divide C2 H6 by 2 to determine the empirical formula CH3. Structural formula in picture given.
To learn more about structural formula use link belwo:
https://brainly.com/question/514499
#SPJ4
Answer:
Both and then Molecular
Explanation:
Which describes an example of ecological succession?

Answers
Answer:
b. a meadow replacing a pond in it's flood plain
Explanation:
I'ts a secondary succession.
A chemist burns a a saltine cracker in a calorimeter containing 2.0 kg of water. The water temperature increases from 25.0°C to 29.7°C.
Calculate the heat content of the cracker in J per cracker.
Hint: you will need the specific heat capacity of water.
Answers
The heat content of the cracker can be calculated using the equation Q = mcΔT, where Q is the heat content, m is the mass of water, c is the specific heat capacity of water, and ΔT is the change in temperature of water.
To calculate Q, we need to first determine the mass of the cracker burned. Let's assume that the mass of the cracker is 1 g. Next, we can calculate the heat transferred from the cracker to the water using the equation Q = mcΔT. We know that m = 2.0 kg (mass of water), c = 4.18 J/g°C (specific heat capacity of water), and ΔT = 29.7°C - 25.0°C = 4.7°C. Plugging these values into the equation, we get ,Q = (2.0 kg)(4.18 J/g°C .4.7°C ,Q = 39.292 J
First, let's use the formula for heat transfer ,q = mcΔT.where q is the heat content, m is the mass of the water, c is the specific heat capacity of water, and ΔT is the change in temperature. Given Mass of water (m) = 2.0 kg Initial temperature (T1) = 25.0°C Final temperature (T2) = 29.7°C Specific heat capacity of water (c) = 4.18 J/g°C, Calculate the change in temperature (ΔT). ΔT = T2 - T1 = 29.7°C - 25.0°C = 4.7°C Convert the mass of water from kg to g.
Mass of water (m) = 2.0 kg × 1000 g/kg = 2000 g.
To know more about mass visit :
https://brainly.com/question/11954533
#SPJ11
24. Which description correctly identifies the substance below?*
5Ca(OH)2
F 5 atoms of calcium, 10 atoms of oxygen, and 10 atoms of hydrogen
G 5 molecules, each containing 1 calcium atom, 2 oxygen atoms, and 2 hydrogen
atoms
O H 8 total atoms
J Both F and G

Answers
Answer:
F is the correct answer
According to the concept of chemical formula, there are 5 atoms of calcium, 10 atoms of oxygen, and 10 atoms of hydrogen.
What is chemical formula?Chemical formula is a way of representing the number of atoms present in a compound or molecule.It is written with the help of symbols of elements. It also makes use of brackets and subscripts.
Subscripts are used to denote number of atoms of each element and brackets indicate presence of group of atoms. Chemical formula does not contain words. Chemical formula in the simplest form is called empirical formula.
It is not the same as structural formula and does not have any information regarding structure.It does not provide any information regarding structure of molecule as obtained in structural formula.
There are four types of chemical formula:
1)empirical formula
2) structural formula
3)condensed formula
4)molecular formula
Learn more about chemical formula,here:
https://brainly.com/question/11995171
#SPJ2
ANSWER THIS QUESTION AND GET 10 POINTS AND BRAINLEST

Answers
Answer:
Its highly basic
what volume of 0.500-m koh(aq) should be added to 100 ml of a buffer solution initially containing 0.13 mol hf (ka
Answers
The volume of 0.500-M KOH(aq) should be added to 100 ml of a buffer solution initially containing 0.13 mol HF and 0.16 mol of NaF is 100 mL of 0.500 M KOH. ka = 6.8 × 10⁴ and pH is 3.59
given that :
ka = 6.8 × 10⁴
and pH is 3.59
moles of HF = 0.13 mol
moles of NaF = 0.16 mol
the pH formula is given as :
pH = pka + log [base]/[acid]
pH = -log(6.8 × 10⁻⁴) + log (0.16 ) / 0.13
pH = 3.33 + 0.0899
pH = 3.5
Thus, the volume of 0.500 M KOH should be added to a buffer solution 0.500 M.
To learn more about buffer solution here
https://brainly.com/question/24262133
#SPJ4
what is the best definition of solar radiation?(Giving brainliest)
-Storing of energy in Earth's oceans
-Energy from the Sun that reaches Earth
-Moisture and heat energy in the atmosphere
-Increased evaporation due to warm water
Answers
Answer:
energy from the sun that reaches earth
Answer:
Energy from the Sub that reaches Earth:)
what volume of a 0.129 m hydrobromic acid solution is required to neutralize 20.9 ml of a 0.194 m sodium hydroxide solution?
Answers
The volume of a 0.129 M hydrobromic acid solution that is required to neutralize 20.9 mL of a 0.194 M sodium hydroxide solution can be calculated using the formula for acid-base titration.
What is acid-base titration?The process of determining the concentration of a known acid or base by reacting it with a solution of unknown concentration is referred to as acid-base titration.
In this chemical reaction, the H+ ions in the acid react with the OH- ions in the base to produce water and a salt.
Hydrobromic acid is an aqueous solution of hydrogen bromide, an extremely corrosive, irritating, and colourless gas. It is a strong acid that dissociates completely in water to form hydronium ions and bromide ions.
What is sodium hydroxide?Sodium hydroxide, also known as lye and caustic soda, is a highly caustic inorganic compound that is commonly used in the chemical industry. It is a white solid that is soluble in water, alcohol, and glycerol.
The reaction between hydrobromic acid and sodium hydroxide is:
HBr + NaOH → NaBr + H2OIn this reaction, 1 mole of hydrobromic acid reacts with 1 mole of sodium hydroxide to produce 1 mole of sodium bromide and 1 mole of water.
Now, let's solve the problem.
The molarity (M) of sodium hydroxide solution is 0.194 M.
The volume (V) of sodium hydroxide solution used is 20.9 mL.
The number of moles (n) of sodium hydroxide used can be calculated using the formula:
n = MV = 0.194 mol/L × (20.9/1000) L = 0.0040566 mol
The number of moles of hydrobromic acid required to neutralize the sodium hydroxide can be calculated using the balanced chemical equation. According to the equation, one mole of hydrobromic acid reacts with one mole of sodium hydroxide.
Therefore, the number of moles of hydrobromic acid required to neutralize 0.0040566 moles of sodium hydroxide is also 0.0040566 mol.
The molarity (M) of hydrobromic acid is 0.129 M.
The volume (V) of hydrobromic acid required can be calculated using the formula:n = MV ⇒ V = n/M = 0.0040566 mol/0.129 mol/L = 0.03146 L = 31.46 mL
Therefore, the volume of a 0.129 M hydrobromic acid solution required to neutralize 20.9 mL of a 0.194 M sodium hydroxide solution is 31.46 mL.
learn more about neutralize here
https://brainly.com/question/23008798
#SPJ11
Arena is investigating a separation technique using the following apparatus. salt solution and/or sugar solution. a) 2 beakers b) tripod stand and wire gauze c) glass rod i) name the separation technique she may be possibly investigating. ii)do you think she has missed out a very important apparatus? if yes, identify and state the importance of the apparatus.
Answers
Arena might be investigating a separation technique known as evaporation, but she is missing a burner to perform it properly.
i) Evaporation is a separation technique that can be used to separate a substance dissolved in a liquid. It is usually applicable when the solvent doesn't have a too high boiling point and the solute is not prone to degradation at increased temperature.
ii) However, as the method requires heating the solution in order to accelerate the evaporation of the solvent, and while the tripod stand and wire gauze are used to mount the beaker with the solvent, they are useless without a burner that is used to introduce heat.
You can learn more about evaporation here:
brainly.com/question/5019199
#SPJ4
You have two pure substances that you cannot identify. Each sample is solid at room temperature. Describe at least five stopes in the appropriate sequence that you wpild take to be able to identify the substance
Answers
Which of the following will conduct electricity?
Question 9 options:
A) Acid solutions only
B) Base solutions only
C) Acid solutions and base solutions
D) Neither acid solutions nor base solutions
Answers
Answer:
I think is c
Explanation:
I hope this helps you
Answer:
Acid solutions and base solutions
Explanation: