Answers
Explanation:
4Al + 3S₂ → 2Al₂S₃
Therefore,
Option B is correct✔.
Related Questions
18. In order to make one molecule of glucose, how many carbon dioxide, ATPs, and NADPH are required?
Answers
To produce one molecule of glucose, 6 molecules of carbon dioxide (\(CO_{2}\)), 18 molecules of adenosine triphosphate (ATP), and 12 molecules of nicotinamide adenine dinucleotide phosphate (NADPH) are required.
Glucose, a six-carbon sugar, is synthesized through the process of photosynthesis in plants. It involves the Calvin cycle, which incorporates carbon dioxide, ATP, and NADPH to produce glucose. For each molecule of glucose formed, 6 molecules of carbon dioxide are required.
The energy needed for glucose synthesis is provided by ATP, which is an energy-rich molecule. In the Calvin cycle, the synthesis of one glucose molecule requires 18 molecules of ATP.
NADPH, a coenzyme involved in energy transfer reactions, is required for the reduction of carbon dioxide during the Calvin cycle. In the process, 12 molecules of NADPH are utilized to produce one molecule of glucose. These components play crucial roles in capturing and storing energy, as well as providing carbon atoms for the formation of glucose, which serves as a vital energy source for organisms.
Learn more about Calvin cycle here:
https://brainly.com/question/26846190
#SPJ11
(a) The original value of the reaction quotient, Qc, for the reaction of H2(g) and I2(g) to form HI(g) (before any reactions take place and before equilibrium is established), was 5.56. On the following graph, plot the points representing the initial concentrations of all three gases. Label each point with the formula of the gas.
Answers
Answer:
Here's what I get
Explanation:
Assume the initial concentrations of H₂ and I₂ are 0.030 and 0.015 mol·L⁻¹, respectively.
We must calculate the initial concentration of HI.
1. We will need a chemical equation with concentrations, so let's gather all the information in one place.
H₂ + I₂ ⇌ 2HI
I/mol·L⁻¹: 0.30 0.15 x
2. Calculate the concentration of HI
\(Q_{\text{c}} = \dfrac{\text{[HI]}^{2}} {\text{[H$_{2}$][I$_{2}$]}} =\dfrac{x^{2}}{0.30 \times 0.15} = 5.56\\\\x^{2} = 0.30 \times 0.15 \times 5.56 = 0.250\\x = \sqrt{0.250} = \textbf{0.50 mol/L}\\\text{The initial concentration of HI is $\large \boxed{\textbf{0.50 mol/L}}$}\)
3. Plot the initial points
The graph below shows the initial concentrations plotted on the vertical axis.

The initial concentration of the HI at the time 0 sec, has been 0.05 mol/L.
The balanced chemical equation for the reaction has been:
\(\rm H_2\;+\;I_2\;\rightarrow\;2\;HI\)
The given initial concentration of hydrogen has been 0.030M, and the given concentration of iodine has been 0.015 M.
The reaction quotient for the following reaction has been:
Reaction quotient = \(\rm \dfrac{[HI]^2}{[H_2]\;[I_2]}\)
The concentration of HI for the reaction quotient 5.56 has been:
5.56 = \(\rm \dfrac{[HI]^2}{[0.030]\;[0.015]}\)
\(\rm [HI]^2\) = 0.0025 M
HI = 0.05 mol/L or 0.05 M.
The concentration of the HI at the initial concentration has been 0.05 mol/L.
The graph attached has been plotted as the concentration as the function of time.
The initial concentration of hydrogen, iodine, and hydrogen iodide have been plotted as the function of time.
For more information about the reaction quotient, refer to the link:
https://brainly.com/question/8205004

Question 8 (5 points)
What is the pH of a solution with a 4.19 x 105 M hydronium ion concentration?
Answers
[H+][OH-]=10-¹⁴
We are using this formula because we need to find the H+
substitute the value given for hydronium ion for OH-
[H+][4.19×10⁵]=10-¹⁴
[H+]=10-¹⁴÷4.19×10⁵
[H+]=2.387×10-¹⁹
Then the pH of the solution will be
pH= –log¹⁰ [H+]
pH = –log¹⁰ [2.387×10-¹⁹]
pH= –log¹⁰2.387+19log¹⁰
= –0.378+19
pH =18.622
what volume of methane gas, ch4, reacts to give 5.00 ml of carbon dioxide gas? (assume temperature and pressure remain constant.) ch4(g) o2(g) spark co2(g) h2o(g)
Answers
Carbon dioxide is produced when 5 mL of methane gas interacts.
Methane, a hydrocarbon, is the primary component of natural gas. Methane has an effect on the climate and temperature of the earth because it is a greenhouse gas (GHG). Methane is released into the atmosphere through a variety of natural and anthropogenic (caused by humans) sources.
Methane is non-toxic and safe to breathe in small amounts, but if it is allowed to replace air in large quantities, the absence of oxygen could result in suffocation.
\(CH_{4}(g) + 2O_{2} \rightarrow CO_{2} + 2H_{2}O(g)\)
1 volume of \(CH_{4}\) = 1 volume of \(CO_{2}\)
5 ml of \(CH_{4}\) = 1 × 5 mL CO2
= 5 mL CO2
So, volume of methane = 5mL
Learn more about methane gas:
brainly.com/question/12645626
#SPJ4
Help Help qwq
What are some opportunities of background chemistry?
Answers
Answer:
Analytical Chemist.
Chemical Engineer.
Chemistry Teacher.
Forensic Scientist.
Geochemist.
Hazardous Waste Chemist.
Materials Scientist.
Pharmacologist.
Explanation:
When European demand for a certain solvent declined, Dow Chemical instructed its German plant to switch to manufacturing a chemical that had been imported from Louisiana and Texas. Dow Chemical would be best described as a(n)
Answers
Dow Chemical would be best described as a multinational corporation in this scenario.
What is a Multinational corporation?
This is the type of corporation which are involved in controlling production of goods and services in at least one country different from that of residence.
Dow chemical is known to have a plant in Germany and Russia thereby making it a multinational corporation.
Read more about Multinational corporation here https://brainly.com/question/494475
#SPJ1
4. A gas occupying a balloon with a volume of 8.29 L and at 752 mmHg and 23 C is released into the
atmosphere. If the balloon expands to 11.39 L at a pressure of 358 mmHg, what is the temperature of the gas
in C?
YOU WILL RECEIVE BRAINLIEST IF YOU ANSWER CORRECLTY!!
Answers
Ideal gas law is valid only for ideal gas not for vanderwaal gas. Combined Boyle's and Charles' gas law is used here. The new temperature of gas is 79.3°C.
What is ideal gas equation?Ideal gas equation is the mathematical expression that relates pressure volume and temperature.
Mathematically,
PV=nRT
where,
P = pressure of gas
V= volume of gas
n =number of moles of gas
T =temperature of gas
R = Gas constant = 0.0821 L.atm/K.mol
Combining Boyle's and Charles' gas law
P₁V₁/T₁ = P₂V₂/T₂
{ (752 mmHg) (8.29 L )} ÷296K ={ ( 358 mmHg) (11.39 L)} ÷T₂
T₂ = 79.3°C
Therefore the new temperature of gas is79.3°C.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ1
At -32.7 °C, a gas takes up 0.750 mL. What temperature, in °C, would be needed to reduce the volume to half that amount?
Answers
The combined gas law formula is given as:
P1 * V1 / T1 = P2 * V2 / T2
Where:
P1 = Initial pressure
V1 = Initial volume
T1 = Initial temperature
P2 = Final pressure
V2 = Final volume
T2 = Final temperature
Given:
Initial volume, V1 = 0.750 mL
Final volume, V2 = 0.750 mL / 2 = 0.375 mL
Initial temperature, T1 = -32.7 °C
Let's denote the final temperature, T2, as the unknown value we are trying to find.
We can rearrange the formula to solve for T2:
T2 = (P2 * V2 * T1) / (P1 * V1)
We need to determine the final pressure, P2, in order to use this formula. However, the problem statement does not provide information about pressure. Without knowing the pressure, we are unable to determine the exact temperature needed to reduce the volume to half of the initial amount. Additional information, such as the pressure, is required to solve this problem.
The following statements were taken from the procedures of four
different investigations. The statement from which investigation is an
example of repetition?
Answers
Answer: D. Investigation 4
Answer:
Investigation D
Explanation:
The electron dot diagram for a neutral atom of chlorine (atomic number 17) is shown below.
Which of the following symbols represents a chlorine ion with a stable arrangement of eight valence electrons?
A. 35Cl1-
B. 35Cl2-
C. 35Cl1+
D. 35Cl
Answers
Answer:
A. 35Cl1-
Explanation:
Chlorine needs 1 more electron to have full octet thus will take 1 electron and possess a -1 charge.
. the density (d) of a substance is an intensive property that is defined as the ratio of its mass (m) to its volume (v). density
Answers
Density is intensive because it is the ratio of two extensive properties that is mass to volume.
What is Density ?Density is defined as mass per unit volume. S.I unit if density is kg/m³.
It is expressed as
Density = \(\frac{\text{Mass}}{\text{Volume}}\) or \(d = \frac{m}{V}\)
What is Intensive Property ?Intensive property is the property which does not depend on the size of the system. Intensive property can be easily identified. Intensive property is the independent property. The size does not change in this.
Example of intensive property are Density, Freezing point, melting point, colour, Lustre, etc.
What is Extensive property ?Extensive property is the property which depend on the substance. Extensive property cannot be easily identified.
Example of extensive property length, weight, volume, mass etc.
Thus from the above conclusion we can say that Density is intensive because it is the ratio of two extensive properties that is mass to volume.
Learn more about Density here: https://brainly.com/question/1354972
#SPJ4
Disclaimer: The given question is incomplete on the portal. Here is the complete question.
Question: The density (d) of a substance is an intensive property that is defined as the ratio of its mass (m) to its volume (v)
density = \(\frac{\text{Mass}}{\text{Volume}}\) or \(d = \frac{m}{V}\)
Considering that mass and volume are both extensive properties, explain why their ratio, density is intensive.
Select the compound that is soluble in water?

Answers
Answer:
NaOH
Explanation:
NaOH is among the water soluble bases
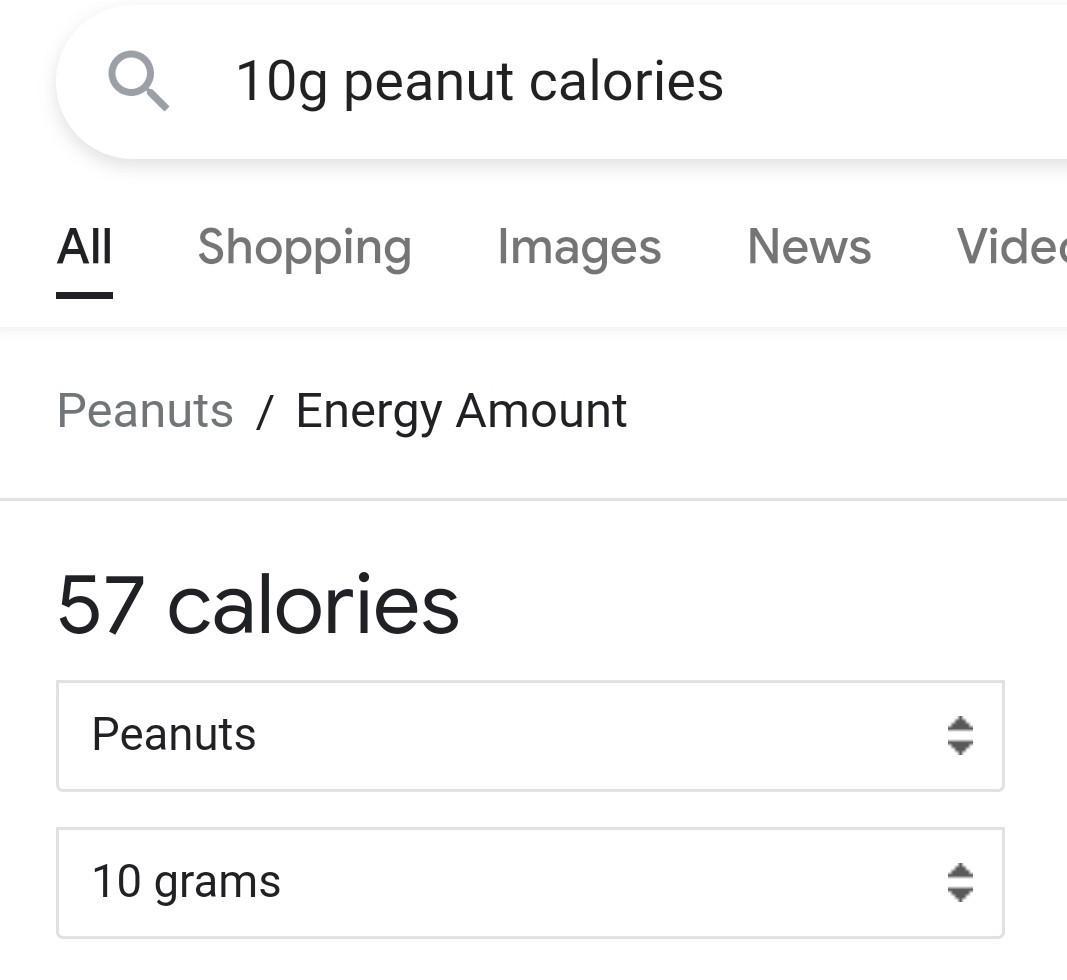
how many calories is in one peanut if the volume of water is 10 mL and the water temperature is rise 4 degrees celsius
Answers
Answer:
40.02 calories
Explanation:
V = 10 mL = 10g
we know t went up by 4°C, this is our ∆t as it is a change.
Formula that ties it together: Q = mc∆t
where,
Q = energy absorbed by water
m = mass of water
c = specific heat of water (constant)
∆t = temperature change
Q = (10 g) x (4.186 J/g•°C) x (4°C)
Q = 167.44 J
Joules to Calories:
167.44 J x 1 cal/4.184 J = 40.02 calories
(makes sense as in image it is close to the value).
When forming an ionic bond, how does the configuration of valence electrons change
Answers
Answer:
Ionic bonds are a class of chemical bonds that result from the exchange of one or more valence electrons from one atom, typically a metal, to another, typically a nonmetal. This electron exchange results in an electrostatic attraction between the two atoms called an ionic bond.
Explanation:
Answer:
The electrons are transferred from one atom to another. The atom that loses electrons becomes positive.
Explanation:
I don't really understand what form of an answer you need but I hope this helps?
What is the Ka of a 1.9 ~ 10-2 M
solution of carbonic acid (H2CO3)
with a pH of 3.88?
Ka = [ ? ] × 10!?)
Helllllp
Answers
Answer:
Ka = 9.2x10⁻⁷
Explanation:
The equilibrium of carbonic acid in water is:
H₂CO₃ ⇄ HCO₃⁻ + H⁺
Where Ka is defined as:
Ka = [HCO₃⁻] [H⁺] / [H₂CO₃]
The equilibrium concentration of the species is:
[H₂CO₃] = 1.9x10⁻² - X
[HCO₃⁻] = X
[H⁺] = X
As pH is -log[H⁺]
3.88 = -log[H⁺]
1.318x10⁻⁴ = [H⁺] = X
Replacing:
[H₂CO₃] = 1.9x10⁻² - 1.318x10⁻⁴ = 1.8868x10⁻²
[HCO₃⁻] = 1.318x10⁻⁴
[H⁺] = 1.318x10⁻⁴
Replacing in ka equation:
Ka = [1.318x10⁻⁴] [1.318x10⁻⁴] / [1.8868x10⁻²]
Ka = 9.2x10⁻⁷Answer: 9.2 x 10^-7
Explanation:
7. According to the law of conservation of mass, the mass of the Must be equal to the mass of the
Answers
Answer:
According to the law of conservation of mass, the mass of the reactants must be equal to the mass of the products
Explanation:
law of conservation of mass
If a laser operating at a wavelength of 488 nm and a power of 123.0 mW is turned on for 18.73 minutes, how many photons has it emitted?
Answers
Answer:
3.39e+20
Explanation:
A hammer strikes on a nail with a force of 144 N downward. What is the force that the nail applies to the hammer?
Answers
Answer:
144 N
Explanation:
Newton's 3rd Law: "For every action, there is an equal and opposite reaction."
How does the temperature change when a layer of glass is added?
Answers
Answer:
thermal shock
Explanation:
the temperatures inside the glass jar should have continued to increase over time. Internal stresses due to uneven heating. This is also known as “thermal shock”.
In general, the thicker the glass, the more prone it will be to breaking due to the immediate differences in temperature across the thickness of glass.
Borosilicate glass is more tolerant of this, as it has a higher elasticity than standard silicon glass.
You may also note that laboratory test tubes and flasks are made with thinner walls, and of borosilicate glass, when designated for heating.
answer the pic below

Answers
Answer: 18
Explanation:
i think so
Old photographic flashbulbs burn magnesium metal in a reaction that causes a flash of brilliant white light. A photographer would need to take care not to suffer burned fingers when trying to replace the hot bulb. What best identifies the energy transformation that occurs in the flashbulb? chemical energy to electromagnetic energy and thermal energy electromagnetic energy to chemical energy and thermal energy chemical energy to electromagnetic energy thermal energy to chemical energy
Answers
Answer:
A
Explanation:
Answer:
A
Explanation:
Not D or B
Determine the number of grams in a mole of each of the following gases. (Pay attention to gases that have diatomic molecules.)
(a) carbon monoxide
____________ g
(b) helium
_____________g
(c) nitrogen
_____________g
Answers
The mole is a unit used to measure the amount of a substance. One mole of any substance is defined as the amount of that substance that contains the same number of particles (such as atoms, molecules, or ions) as there are in 12 grams of carbon-12. This number of particles is known as Avogadro's number, which is approximately 6.022 x 10^23 particles per mole.
The molar mass of a substance is the mass of one mole of that substance and is expressed in grams per mole. To determine the number of grams in a mole of a substance, you simply need to calculate the molar mass of the substance by adding up the atomic masses of each atom in its chemical formula.
For example, for carbon monoxide (CO), the atomic mass of carbon is 12.01 g/mol, and the atomic mass of oxygen is 16.00 g/mol. Adding these together gives a molar mass of 28.01 g/mol, which means that one mole of CO has a mass of 28.01 grams.
For helium (He), the atomic mass is 4.00 g/mol, so one mole of helium has a mass of 4.00 grams.
For nitrogen (N2), the atomic mass of nitrogen is 14.01 g/mol, and since there are two nitrogen atoms in each molecule, the molar mass of nitrogen gas is 28.02 g/mol. Therefore, one mole of nitrogen gas has a mass of 28.02 grams.
To know more about mole,
https://brainly.com/question/31123980
#SPJ11
2.00 gram of a compound requires the following quantities of solvent to dissolve: 90.0 ml of water, 7.50 ml of chloroform, 32.0 ml of diethyl ether, or 85.0 ml of benzene. calculate the partition coefficient of the compound between chloroform and water, diethyl ether and water, and benzene and water. which solvent would you choose to extract the compound from an aqueous solution?
Answers
The solvent that would be used for extracting the compound from an aqueous solution will be chloroform as it has maximum solubility.
What is solubility?
Solubility is a degree to which the a substance dissolves in a solvent to make a solution. Solubility is expressed as grams of solute per litre of solvent.
Solvent requires 2 grams of compound to dissolve.
Finding solubility
For water, 2g/90ml= 2.22
For chloroform, 2g/7.50= 26.6
For diethyl ether, 2g/32= 6.25
For benzene, 2g/85= 2.35
Partition Coefficients -
For chloroform = 11.98
For Diethyl Ether = 2.81
For Benzene = 1.05
Hence, Chloroform would be chosen to extract compound from an aqueous solution as it has maximum solubility.
To know more about solubility follow the link
https://brainly.com/question/24057916
#SPJ4
Since chloroform has the greatest solubility, it would be the solvent of choice for removing the compound from an aqueous solution.
What is solubility?
A homogeneous mixture of one or even more solutes in a solvent is referred to as a solution. A typical illustration of a solution is the addition of sugar cubes to a cup of tea or coffee. Solubility is a quality that aids in the dissolution of sugar molecules. Thus, the ability of a substance (solute) to dissolve in a specific solvent can be defined as solubility. Any substance that is dissolved in a solvent and is either solid, liquid, or gas is referred to as a solute.
Compound needs 2 grammes of solvent to dissolve it.
Finding solubility
For water, 2g/90ml= 2.22
For chloroform, 2g/7.50= 26.6
For diethyl ether, 2g/32= 6.25
For benzene, 2g/85= 2.35
Partition Coefficients -
For chloroform = 11.98
For Diethyl Ether = 2.81
For Benzene = 1.05
As a result of its high solubility, chloroform would be used to extract a compound from an aqueous solution.
To know more about solubility follow the link
brainly.com/question/24057916
#SPJ4
a liquid solvent is added to a flask containing an insoluble solid. the total volume of the solid and liquid together is 91.0 ml. 91.0 ml. the liquid solvent has a mass of 21.0 g 21.0 g and a density of 0.865 g/ml. 0.865 g/ml. determine the mass of the solid given its density is 1.75 g/ml.
Answers
The mass of the solid of density 1.75 g/ mole is 116.77g. This is calculated using the expression for density.
Density is defined as the mass per unit of volume of the substance. The symbol used for density is ρ. Basically density is defined as mass divided by volume.
Density = mass/ volume
mass of the liquid = 21.0 g
density of the liquid = 0.865 g/ mole
volume of liquid = mass / density
= 21.0 g / 0.865g/ mole
= 24.27 ml
The total volume of the solid and liquid together is 91.0 ml.
Total volume = volume of solid + volume of liquid
volume of solid = total volume - volume of liquid
= 91.0ml - 24.27 ml
= 66.73 ml
density of the solid= 1.75 g/ mole.
mass = density * volume
= 1.75 g /mole * 66.73 ml
= 116.77 g
To learn more about Density please visit:
https://brainly.com/question/1354972
#SPJ4
Polyelectrolytes are typically used to separate oil and water in industrial applications. The separation process is dependent on controlling the pH. Fifteen (15) pH readings of wastewater following these processes were recorded. Is it reasonable to model these data using a normal distribution? 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 10.0 10.5 7.6 11.4 11.4 10.0 Yes, it passes the "fat pencil" test. Therefore, a normal distribution is a reasonable model. No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O Yes, it passes the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O No, it does not pass the "fat pencil" test. Therefore, a normal distribution is a reasonable model.
Answers
No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. Option B is the correct answer.
The "fat pencil" test is a quick visual check to determine if a dataset can be reasonably approximated by a normal distribution. In this case, the pH readings of wastewater show a significant deviation from a normal distribution. The presence of several low pH values (1.0) and a few high pH values (10.0, 10.5, 11.4) indicate a non-normal distribution with skewness and potential outliers. Therefore, it is not reasonable to model these data using a normal distribution.
Option B is the correct answer.
You can learn more about normal distribution at
https://brainly.com/question/4079902
#SPJ11
According to the
graph, what happens
to the concentration
of A over time?
Concentration (M)
Reaction: 2A A₂
Time (sec)
A. It decreases and then levels out.
B. It decreases consistently.
C. It increases and then levels out.
D. It increases consistently.
Answers
The concentration of A decreases and then levels out. Option A
How does concentration of the reactant change?
In many chemical reactions, a reactant is consumed as the reaction progresses, leading to a decrease in its concentration over time. The reactant molecules are transformed into products, and as the reaction proceeds, the concentration of the reactant gradually diminishes.
At equilibrium, the concentrations of both reactants and products remain relatively constant over time, although they can coexist.
Learn more abaout reactant:https://brainly.com/question/30129541
#SPJ1
Good scientific design involves replication and repetition.
is the act of performing a task several times.
is the ability of a process to be repeated in the same manner by another individual.
Answers
Good scientific design involves a. replication and repetition, which is fundamental to gaining understanding by using the scientific method.
What do replication and repetition mean in scientific design?Replication and repetition are fundamental in scientific design because they allow for obtaining results that can be tested by using the scientific method and thus validated or refute in the light of new scientific evidence.
Therefore, with this data, we can see that replication and repetition are critical for a good scientific design in order to obtain reproducible results that can be used to develop theories.
Learn more about replication and repetition here:
https://brainly.com/question/11411764
#SPJ1
What is the claim in a literary analysis?
a reason that makes your opinion believable
an emotional statement of opinion
a reasonable, debatable opinion about the work
a summary of the factual evidence
Answers
An argumentative, plausible view of the literary work being evaluated is the claim in a literary analysis.
The claim in a literary analysis, which is an interpretation of a literary work, is the author's argument or viewpoint regarding the relevance or meaning of the work.
The assertion needs to be clear, debatable and backed up by textual evidence.
Literary analysisThe claim in literary analysis is the main viewpoint or argument that the author is advancing regarding the relevance or meaning of the literary work under consideration.
The assertion should be a reasonable, disputed opinion that can be backed up by textual evidence, and it should be sufficiently detailed to be convincing and understandable to the reader.
For instance, in an interpretation of William Shakespeare's play "Hamlet," a writer can contend that, rather than a lack of courage, Hamlet's hesitation to exact revenge on his father's murderer stems from his desire for justice and his battle with indecision.
This assertion is both plausible and problematic because different readers or critics may interpret Hamlet's actions differently.
learn more about the literary analysis here
https://brainly.com/question/9965425
#SPJ1
which statement best defines a cell?
an independent functional unit of life
a crystal-like structure that exists in all living things
the smallest organelle of all living organisms
the basic unit of structure and function in all living things
Answers
the basic unit of structure and function in all living things.
Answer:
all living things.
Explanation:
Transcribed Image Text:Which of the following ketones would be the best choice to use for a crossed aldol reaction with butanal? A B O a. Ketone C O b. Ketones B and C work equally well C. Ketone A d. Ketones A and B work equally well е. Ketone B
Answers
The best choice of ketone to use for a crossed aldol reaction with butanal would be ketone B.
The best choice of ketone to use for a crossed aldol reaction with butanal would be ketone B. This is because ketone B has a good leaving group and is capable of undergoing the aldol reaction. The aldol reaction involves the condensation of an aldehyde or ketone with an enolate to form a β-hydroxy carbonyl compound. In this case, the enolate is derived from butanal, and the ketone is the other reactant. Ketones A and C have a poor leaving group and are not suitable for the aldol reaction. Ketones B and C have similar structures, but ketone B is more reactive due to the presence of a methyl group, which stabilizes the enolate intermediate. Therefore, ketone B is the best choice for the crossed aldol reaction with butanal.
To know more about aldol reaction visit: https://brainly.com/question/29563033
#SPJ11
