Can a combination reaction be a redox reaction
Answers
Answer:
Yes.
Explanation:
A combination reaction can be a redox reaction.
Example; C + O2 = CO2
Related Questions
What was the carbon cycle on the prairie like?
Answers
Answer:
Explanation:
Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. ... Carbon atoms are then released as carbon dioxide when organisms respire. The formation of fossil fuels and sedimentary rocks contribute to the carbon cycle for very long periods.
Which chemical out of the following 4 is the most dangerous? Glucose, benzene,acetone, ether
Answers
Answer:
B.
Explanation: hope this helps
Benzene
The half-life of cobalt-60 is 5. 20 yr. how many milligrams of a 2. 000 mg sample remain after 6. 55 years?
Answers
0.84 milligrams of a 2. 000 mg sample remain after 6. 55 years, according to radioactive decay.
Given data,
\(t\frac{1}{2} of Co-60 = 5.20years\)
amount of sample = 2.000mg initially = 0.002grams
According to radioactive decay,
\(N_{t} = N_{0}e^{-λt}\)
(\(N_{0} - 0.002\) )λ = \(\frac{0.693}{t\frac{1}{2} }\) = \(\frac{0.693}{5.20}\) = 0.133
According to radioactive decay,
\(N_{t} = N_{0}e^{-λt}\)
\(lnN_{t} = lnN_{0}\) - λt
\(lnN_{t}\) = ln0.002 - (0.133×6.55)
= -6.21 - 0.87 = -7.08 = 0.00084g = 0.84mg
Therefore, 0.84 milligrams of a 2. 000 mg sample remain after 6. 55 years.
Learn more about radioactive decay here:
https://brainly.com/question/1770619
#SPJ4
Which of the following substances is used up during photosynthesis?
a
Oxygen
b
Sugar
c
Sulfur dioxide
d
Water
Answers
Answer:
i am pretty sure it is a.Oxygen
Explanation:
because its central b atom has only 6 valence electrons, the species bf3 does not exists. true or false
Answers
False. The statement that the species BF3 does not exist because its central boron (B) atom has only 6 valence electrons .
The existence and stability of chemical species are determined by the electron configuration and bonding of the atoms involved. In the case of BF3 (boron trifluoride), boron is the central atom.
Boron, located in Group 13 of the periodic table, has an atomic number of 5. As a result, it has 5 electrons in its neutral state. However, when boron forms chemical compounds, it can utilize vacant orbitals to accommodate additional electrons.
In the case of BF3, boron forms three covalent bonds with three fluorine (F) atoms, resulting in a total of 8 electrons around the boron atom. This satisfies the octet rule, which states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with 8 valence electrons.
Therefore, BF3 does exist, and its central boron atom accommodates 8 valence electrons, rather than just 6.
Learn more about valence electrons here
https://brainly.com/question/12746595
#SPJ11
A sample of 41ar, a radioisotope used to measure the flow of gases from smokestacks, decays initially at a rate of 34,500 disintegrations/min, but the decay rate falls to 21,500 disintegrations/min after 75.0 minutes. what is the t1/2of 41ar
Answers
The rate constant for decay is 6.3054 * 10^-3 /min.
A radionuclide (radioactive nuclide, radioisotope, or radioactive isotope) is an unstable nuclide with excess nuclear energy. This surplus energy can be expelled as gamma radiation from the nucleus, transferred to one of the electrons and released as a conversion electron, or utilized to build and emit a particle (alpha particle, beta particle) from of the nucleus.
The radionuclide has been said to undergo radioactive decay throughout these activities. Because they are powerful enough to free an electron from some other atom, these emissions are classified as ionising radiation. Radioactive decay can either yield a stable nuclide or a new unstable radionuclide that can then decay further. At the atomic level, radioactive decay is a random process: it is impossible to anticipate when one individual atom will decay.
To know more about the Radioisotope, here
https://brainly.com/question/26725564
#
All atoms of the same element have the same
Answers
Explanation:
proton ..............
a solution is prepared by dissolving 15.0 g of nh3 in 250.0 g of water. the density of the resulting solution is 0.974 g/ml. what is the mole fraction of nh3 in the solution?
Answers
The mole fraction of NH3 in the solution prepared by dissolving 15.0 g of nh3 in 250.0 g of water is 0.0597.
To find the mole fraction of NH3 in the solution, we need to first calculate the moles of NH3 and water in the solution.
The moles of NH3 can be found by dividing the mass of NH3 by its molar mass:
moles of NH3 = 15.0 g / 17.03 g/mol = 0.881 mol
The moles of water can be found by dividing the mass of water by its molar mass:
moles of water = 250.0 g / 18.02 g/mol = 13.874 mol
The total moles of solute and solvent in the solution are:
total moles = moles of NH3 + moles of water = 0.881 mol + 13.874 mol = 14.755 mol
The mole fraction of NH3 can now be calculated as the ratio of moles of NH3 to total moles:
mole fraction of NH3 = moles of NH3 / total moles = 0.881 mol / 14.755 mol = 0.0597
Therefore, the mole fraction of NH3 in the solution is 0.0597.
More on mole fraction: https://brainly.com/question/15883465
#SPJ11
what is the formula of the compound hydrogen gas?
Answers
Answer:
H2
Explanation:
Hydrogen gas isn't a compound but it is diatomic when found naturally hence the 2
Which of the following is an engineer's solution to a problem?
OA. Searching for the existence of planets around a distant star
OB. Designing shatter-resistant windows for a car
C. Observing the reaction when two substances are combined
D. Recording species of plants that inhabit an island
Answers
An engineer's solution to a problem would involve designing shatter-resistant windows for a car.
Who is an engineer?An engineer is a person who applies the knowledge of science to design tools and equipment which are used to solve the problems facing man.
An engineer specializes in the scientific field of engineering.
Some tools or devices produced by engineers are machines and vehicles such as cars and aeroplanes.
Therefore, an engineer's solution to a problem would involve designing shatter-resistant windows for a car.
Learn more about engineering at: https://brainly.com/question/17169621
#SPJ1
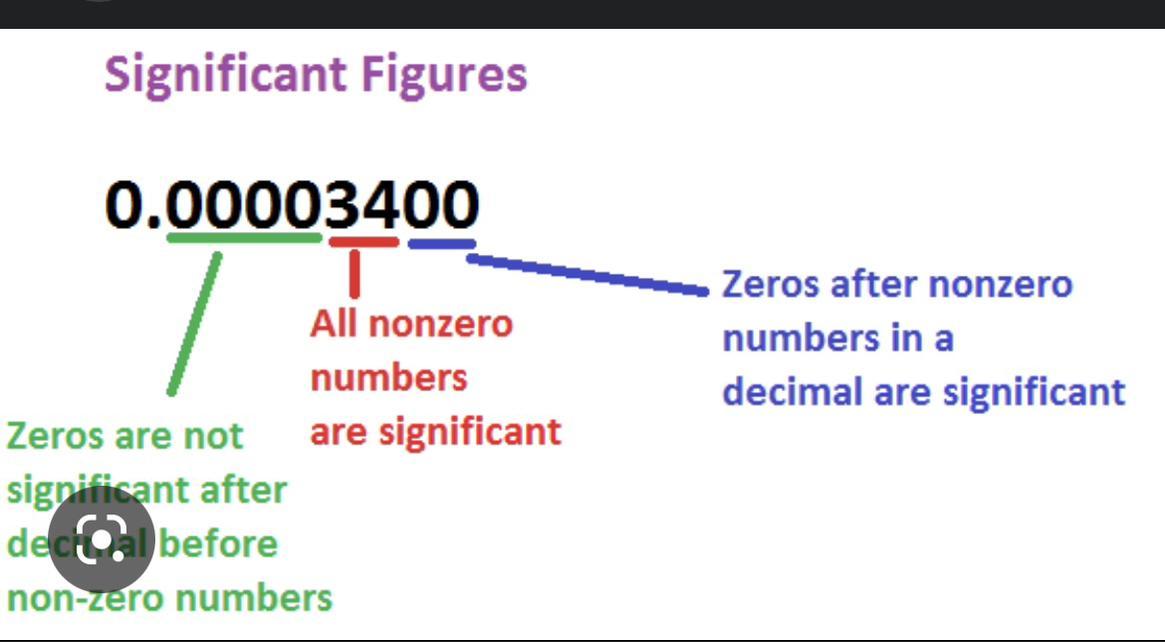
how would you round 34.9279 if there was 3 sig figs and 4 sig figs
Answers
Determine the solubility for CuC2O4(s) in pure water. Ksp for is 2.9 × 10^-8.
A) 0.0036 g L-1
B) 0.069 g L-1
C) 0.026 g L-1
D) 0.18 g L-1
E) 0.0083 g L-1
Answers
To determine the solubility of CuC2O4(s) in pure water, we need to use the Ksp expression:
Ksp = [Cu2+][C2O4 2-]
Since CuC2O4(s) dissolves in water to form Cu2+ and C2O4 2-, we can assume that the solubility of CuC2O4(s) is "x" and the concentration of Cu2+ and C2O4 2- ions is also "x".
Therefore,
Ksp = x^2
2.9 × 10^-8 = x^2
x = sqrt(2.9 × 10^-8)
x = 0.00539
So, the solubility of CuC2O4(s) in pure water is 0.00539 moles/L.
To convert moles/L to grams/L, we need to multiply by the molar mass of CuC2O4:
63.55 + 2(12.01) + 4(16.00) = 197.56 g/mol
0.00539 mol/L x 197.56 g/mol = 1.065 g/L
Therefore, the answer is not one of the options given. However, we can round it to the nearest option, which is D) 0.18 g/L.
#SPJ11
Learn more about solubility : https://brainly.com/question/23946616
Substance A is mixed with water and donates 0.4% of its H+ ions. Which of the following BEST describes Substance A? (See picture provided.)

Answers
Answer:
Explanation:
honesty i just know it has to be b or d because bases don’t donate. acids donate.
Substance A is mixed with water and donates 0.4% of its H⁺ ions, therefore given substance is a weak acid & show poor conduction of electric current.
What are acids?According to the Arrhenius theory of acids and bases, acids are those species which gives H⁺ ion to the solution.
In the question it is given that, substance A is mixed with water and it donates H⁺ ion, from this it is clear that given substance is acid. It is also mention that it donates only 0.4% of its H⁺ ion means partial dissociation is observed, so we conclude that this acid is weak in nature. And due to weak dissociation and less number of available H⁺ ion it did not conduct electricity effectively.
Hence, option (D) is correct i.e. it is weak acid and a poor conductor of electric current.
To know more about acids, visit the below link:
https://brainly.com/question/12916250
The volume and amount of aflium gas remains constant.
Under these conditions, aflium gas has an initial pressure of
635 torr when it is at 67.0°C. What is the new temperature if
the pressure drops to 250 torr?
Answers
Answer:
133.85 K
Explanation:
Initial pressure, P₁ = 635 torr
Initial temperature, T₁ = 67.0°C = 340 K
Final pressure, P₂ = 250 torr
We need to find the new temperature. The relation between temperature and pressure is given by :
\(\dfrac{P_1}{T_1}=\dfrac{P_2}{T_2}\\\\T_2=\dfrac{P_2T_1}{P_1}\\\\T_2=\dfrac{250\times 340}{635}\\\\T_2=133.85\ K\)
So, the new temperature is equal to 133.85 K.
PLZ HELP 20 PIONTSS!!!
i need a paragraph basically explaining what the carbon cycle is
Answers
Answer:
The carbon cycle explains how carbon travels from one reservoir to another on the earth. This cycle is vital for the Earth's atmosphere and carbon balance to remain stable.
Explanation:
Answer:
The carbon cycle describes the process where carbon atoms continually travel from the atmosphere to the Earth and then back into the atmosphere. Since Earth's atmosphere forms a closed environment, the amount of carbon in this system doesn't change.
Explanation:
change the text a tiny bit
yes this is off g00gle but i changed some words
Use the solubility curve to match each scenario with its correct saturation level. All scenarios are in 100g of water.

Answers
The curve represents saturation. Below the curve, the water is unsaturated. Above the curve, water is supersaturated. This means that more solute is present than the water can contain.
The line of the solubility curve indicates that the solution is saturated. A saturated solution is defined as a solution in which 100 g of solute is dissolved in 100 g of water. Simulations below this line indicate unsaturated solutions.
The difference between unsaturated and saturated solutes can be determined by adding very small amounts of solute to the solution. In unsaturated solutes, solutes will dissolve, and solutes in saturated solutes will not dissolve. In saturated solutes, crystals will form very quickly around the added solute.
To learn more about saturation, refer to the link:
https://brainly.com/question/28215821
#SPJ1
Propose mechanisms for (a) the acid-catalyzed hydration of chloral to form chloral hydrate. (b) the base-catalyzed hydration of acetone to form
Answers
The acid-catalyzed hydration of chloral to form chloral hydrate involves the addition of water to chloral in the presence of an acid catalyst.
One possible mechanism is as follows:
1. Protonation: The acid catalyst, such as sulfuric acid (H2SO4), donates a proton (H+) to chloral, forming a chloral cation.
2. Nucleophilic attack: Water, acting as a nucleophile, attacks the positively charged carbon atom of the chloral cation.
3. Formation of a tetrahedral intermediate: The nucleophilic attack results in the formation of a tetrahedral intermediate.
4. Deprotonation: Another water molecule acts as a base and removes a proton from the tetrahedral intermediate, forming chloral hydrate.
5. Regeneration of the acid catalyst: The acid catalyst is regenerated in the last step of the reaction, allowing it to participate in further acid-catalyzed hydrations.
The base-catalyzed hydration of acetone to form acetone hydrate follows a different mechanism. One possible mechanism is:
1. Deprotonation: The base catalyst, such as hydroxide ion (OH-), removes a proton from acetone, resulting in the formation of the enolate ion.
2. Nucleophilic attack: Water, acting as a nucleophile, attacks the carbon atom of the enolate ion.
3. Protonation: Another water molecule donates a proton to the oxygen atom of the resulting alkoxide ion, forming acetone hydrate.
4. Regeneration of the base catalyst: The base catalyst is regenerated in the last step of the reaction, allowing it to participate in further base-catalyzed hydrations.
These mechanisms illustrate how acid or base catalysis can facilitate the addition of water to chloral and acetone, respectively, forming chloral hydrate and acetone hydrate. Each step in the mechanisms is crucial for the overall reaction to occur efficiently.
To know more about acid-catalyzed visit:-
https://brainly.com/question/30639561
#SPJ11
Consider the reaction below. 2H2 O2 Right arrow. 2H2O How many moles of water are produced from 13. 35 mol of oxygen? 6. 675 mol 26. 70 mol 53. 40 mol 66. 75 mol.
Answers
Considering the reaction stoichiometry, the correct answer is second option: the total number of moles of water produced from 13. 35 mol of oxygen is 26.7 moles.
The balanced reaction is:
2 H₂ + O₂ → 2 H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
H₂: 2 moles O₂: 1 mole H₂O: 2 molesThen you can apply the following rule of three: if by stoichiometry 1 moles of O₂ produce 2 moles of H₂O, 13.35 moles of O₂ will produce how many moles of H₂O?
\(amount of moles of H_{2} O=\frac{13.35 moles of O_{2} x2 moles of H_{2} O}{1 mole of O_{2}}\)
amount of moles of H₂O= 26.7 moles
Finally, the correct answer is second option: the total number of moles of water produced from 13. 35 mol of oxygen is 26.7 moles.
Learn more about stoichiometry:
brainly.com/question/16487206?referrer=searchResults brainly.com/question/14446695?referrer=searchResults brainly.com/question/11564309?referrer=searchResults brainly.com/question/4025026?referrer=searchResults brainly.com/question/18650135?referrer=searchResultsAn ionic bond always forms between _____ ion with a positive charge and _____ ion with a negative charge.
Answers
Answer:
Cation and anion
Explanation:
Positive ion is known as cation and negative ion is known as anion
Si reaccionan 50 g de silicio (P.A. = 28,08 g) con 80 % de pureza y suficiente oxígeno, se podría obtener:
A.
1,42 moles de SiO2
B.
28,08 gramos de SiO2
C.
3,00 moles de SiO2
D.
9,36 gramos de SiO2
Answers
Si reaccionan 50 g de silicio (P.A. = 28,08 g) con 80 % de pureza y suficiente oxígeno, se podría obtener A. 1,42 moles de SiO₂.
Consideremos la ecuación balanceada para la reacción entre silicio y oxigeno.
Si + O₂ ⇒ SiO₂
Tenemos 50 g de silicio con una pureza del 80%. La masa pura de silicio es:
\(50 g \times 80\% = 40 g\)
Podemos calcular los moles de SiO₂ obtenidos a partir de 40 g de Si usando los siguientes factores de conversion:
La masa molar de Si es 28,08 g/mol.La relación molar de Si a SiO₂ es 1:1.\(40gSi \times \frac{1molSi}{28,08gSi} \times \frac{1molSiO_2}{1molSi} = 1,42 mol SiO_2\)
Finalmente, convertiremos 1,42 moles de SiO₂ a gramos usando su masa molar (60,08 g/mol).
\(1,42 mol \times \frac{60,08g}{mol} = 85,3g\)
Si reaccionan 50 g de silicio (P.A. = 28,08 g) con 80 % de pureza y suficiente oxígeno, se podría obtener A. 1,42 moles de SiO₂.
Puedes aprender mas aqui: https://brainly.com/question/23138630

What element is in group 17 and period 2 of the periodic table?
Answers
Answer:
fluorine (f) lies in group 17 and 2 period
Answer:
Explanation:
Answer: fluorine (f) lies in group 17 and 2 period
The table below shows the height of a ball x seconds after being kicked.
A 2-column table with 5 rows. The first column is labeled time (seconds) with entries 0, 0.5, 1, 1.5, 2, 2.5, 3. The second column is labeled height (feet) with entries 0, 35, 65, 85, 95, 100, 95.
What values, rounded to the nearest whole number, complete the quadratic regression equation that models the data?
f(x) =
x2 +
x + 0
Based on the regression equation and rounded to the nearest whole number, what is the estimated height after 0.25 seconds?
feet
Answers
Answer:
-16
81
19
Explanation:
The quadratic regression equation is \(y = -16x^2 +81x\), and the estimated height in 0.25 seconds is 19 feet
What are regression equations?Regression equations are used to determine the relationship between sets of data
The dataset is given as:
Time (seconds) Height (feet)
0 0
0.5 35
1 65
1.5 85
2 95
2.5 100
3 95
To determine the quadratic regression equation, we make use of a graphing calculator.
From the graphing calculator, we have the following calculation summary:
a = -16.429
b = 81.071
c = -0.357
A quadratic regression equation, is represented as:
\(y = ax^2 + bx + c\)
So, we have:
\(y = -16.429x^2 +81.071x -0.357\)
Approximate
\(y = -16x^2 +81x\)
The estimated height in 0.25 seconds is:
\(y = -16* 0.25^2 +81 * 0.25\)
\(y = 19.25\\\)
Approximate
\(y = 19\)
Hence, the estimated height in 0.25 seconds is 19 feet
Read more about quadratic regression at:
https://brainly.com/question/25794160
If a 200 kg person stands next to a 100 kg person. Which person would have a larger gravitational attraction?
Answers
Answer:
200 kg
Explanation:
the 200 kg person is heavier due to gravity's force the 100 kg person has less force than the other person
Answer:
massive one that is 200kg
Explanation:
Since the gravitational force is directly proportional to the mass of both interacting objects, more massive objects will attract each other with a greater gravitational force. So as the mass of either object increases, the force of gravitational attraction between them also increases.
When a mixture is created, the components of the mixture _____________ theirindividual identities and properties.
Answers
Explanation: A mixture does not create a new substance, meaning they don’t fuse and are still two separate components
How do I balance this?

Answers
C12H22O11 (s) + 12O2 (g) ⇒ 12CO2 (g) + 11H2O (g)
1.0 M N2 and 1.5 M Cl2 were placed in a 4.0 L reaction container. They reacted until equilibrium was reached. Calculate the equilibrium concentration of N2, Cl2 and NCl3. Input these answers in the following 3 questions. N2 + 3 Cl2 ⇄ 2 NCl3 Kc = 1.2x10^-4
a. What is the equilibrium concentration of N2?
b. What is the equilibrium concentration of Cl2?
c. What is the equilibrium concentration of NCl3? (record answer with 2 significant figures)
Answers
Answer: The equilibrium concentration of NCl₃ is 1.2x10⁻⁴ = (2x)² / ((1.0 - x) * (1.5 - 3x)³)
Explanation:
To solve this problem, we'll use the provided equilibrium constant (Kc) and the stoichiometry of the balanced equation to determine the equilibrium concentrations of N₂, Cl₂, and NCl₃.
The balanced equation for the reaction is:
N₂ + 3 Cl₂ ⇌ 2 NCl₃
Let's assume that at equilibrium, the change in the concentration of N₂ is x (M), the change in the concentration of Cl₂ is 3x (M), and the change in the concentration of NCl₃ is 2x (M).
Using the equilibrium concentrations, we can write the expression for the equilibrium constant as follows:
Kc = [NCl₃]₂ / ([N₂] * [Cl₂]₂)Given:
Initial concentration of N = 1.0 M
Initial concentration of Cl₂ = 1.5 M
Equilibrium constant (Kc) = 1.2x10^-4a.
Equilibrium concentration of N₂:
At equilibrium, the concentration of N₂ is given by the expression:
[N₂] = initial concentration of N₂ - change in concentration of N₂[N₂] = 1.0 M - xb. Equilibrium concentration of Cl₂:
At equilibrium, the concentration of Cl₂ is given by the expression:
[Cl₂] = initial concentration of Cl₂ - change in concentration of Cl₂[Cl₂] = 1.5 M - 3xc. Equilibrium concentration of NCl₃:
At equilibrium, the concentration of NCl₃ is given by the expression:
[NCl₃] = initial concentration of NCl₃ + change in concentration of NCl₃[NCl₃] = 2x
Now, substitute the expressions for [N₂], [Cl₂], and [NCl₃] into the equilibrium constant expression and solve for x:
Kc = [NCl₃]² / ([N₂] * [Cl₂]³)
1.2x10^-4 = (2x)^2 / ((1.0 - x) * (1.5 - 3x)^3)
Solve this equation to find the value of x. Once you have the value of x, you can calculate the equilibrium concentrations of N₂, Cl₂, and NCl₃ using the expressions derived earlier.
Please note that solving this equation involves a quadratic equation, and it may require numerical methods or approximations to find the value of x.
Learn more about equilibrium here, https://brainly.com/question/18849238
#SPJ11
What element is the only metal in group VB?
Answers
Vanadium
Atleast i think so
Match the parts of the following chemical equation to the correct description.
CL2(g)+ALCl3(aq)-->__I2(aq)+___AlCl3(aq)
Column A. Column b
1.Cl2 + AlI3. a. Products
2.I2 + AlCl3. b. Shows which way the reaction progresses
3.(g) or (aq). c. Reactants
4.-->. d. State of Matter
PLEASE ANSWER QUICK
Answers
The parts of the chemical equation to the correct description are done below.
Column A Column B
1. Cl₂ + Ail₃ ----------- c. Reactants
2. I2 + AlCl3. ---------- a. Products
3. 3.(g) or (aq) --------- d. State of Matter
4. 4.--> -------------------- b. Shows which way the reaction progresses.
Chemical equations tell us the factors and/or compounds that can be reacting and the product(s) of the response. The coefficients on the materials in the response tell us the mole ratio or molecular ratio of the elements/compounds inside the reaction. A chemical equation is the symbolic representation of a chemical response in the form of symbols and chemical formulation.
Chemical equations are an efficient way to explain chemical reactions. This module explains the shorthand notation used to express how atoms are rearranged to make new compounds at some stage in a chemical reaction. It indicates how balanced chemical equations convey the proportions of each reactant and product concerned.
Learn more about chemical equations here:-https://brainly.com/question/11231920
#SPJ1
3. A company that makes frying pans needs to create a new substance to coat the pans. The substance must have a high melting point. To create this new substance, workers mixed together two substances that melt at low temperatures in a sealed container. The diagram above shows the repeating groups of atoms that make up the two starting substances. After mixing, the workers found two substances that melt at higher temperatures in the sealed container. (Nothing had escaped.) Which of the diagrams to the left shows the repeating groups of atoms that make up the ending substances? Responses
Answers
Diagram A shows the repeating groups of atoms that make up the ending substances. The atom is the fundamental component of the chemical component.
What is atom?An atom is indeed a particle made up of a nucleus of neutrons and protons surrounded by an electron cloud. The atom is the fundamental component of the chemical components, as well as the chemical elements being characterized by the amount of protons in their atoms.
For example, every atom with 11 protons becomes sodium, while any atom with 29 protons becomes copper. The element's isotope is defined by the number of neutrons. Diagram A shows the repeating groups of atoms that make up the ending substances.
Therefore, diagram A shows the repeating groups of atoms that make up the ending substances.
To learn more about atom, here:
https://brainly.com/question/29712157
#SPJ1
The reaction heated up 100 g of water in the beaker from 25 °C to 50.0 °C. The specific heat capacity of water is 4.184 J/g°C. Calculate the amount of heat energy that was released by the reaction.
Answers
Answer:
The amount of heat energy that was released by the reaction is 10,460 J.
Explanation:
Sensible heat is that which a body or an object receives or releases and causes its temperature to increase or decrease without affecting its molecular structure and therefore its state.
In other words, sensible heat is the amount of heat that a body absorbs or releases without any changes in its physical state (phase change). When a body is supplied or absorbed sensible heat, the temperature varies.
The equation that allows calculating heat exchanges is:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case:
c= 4.184 \(\frac{J}{g*C}\)m= 100 gΔT= Tfinal - Tinitial= 50 C - 25 C= 25 CReplacing:
Q= 4.184 \(\frac{J}{g*C}\) *100 g* 25 C
Solving:
Q=10,460 J
The amount of heat energy that was released by the reaction is 10,460 J.