Describe two situations in which you observed or performed an energy transformation. For each situation, name the starting form of energy and an ending form of energy.
Answers
Answer:
The Sun transforms nuclear energy into heat and light energy.
Our bodies converting chemical energy in our food into mechanical energy for us to be able to move.
An electric fan transforming electrical energy into kinetic energy.
Related Questions
When copper wire is placed into a silver(I) nitrate solution, silver crystals and copper(I) nitrate solution form. If a 20.0g sample of copper is used, determine the theoretical yield of silver. If 60.0g silver is actually recovered, determine the percent yield of the reactions.
Answers
Answer:
Theoretical yield of silver = 67.97 g Ag
Percent yield of the reaction = 88.3%
Explanation:
This reaction forms copper(II) nitrate instead of copper(I) nitrate.
First, we have to write the balanced chemical equation for the reaction of copper (Cu) with silver(I) nitrate (AgNO₃) to give silver (Ag) and copper(I) nitrate (CuNO₃):
Cu(s) + 2AgNO₃(aq) → 2Ag(s) + Cu(NO₃)₂(aq)
From the equation, 1 mol of Cu(s) produces 2 moles of Ag(s). We convert the moles to mass with the molecular weight (MW) of the compounds:
MW(Cu) = 63.5 g/mol
mass of Cu = 1 mol Cu x 63.5 g/mol = 63.5 g Cu
MW(Ag) = 107.9 g/mol
mass of Ag = 2 mol Ag x 107.9 g/mol = 215.8 g Ag
Thus, we have the conversion factor: 215.8 g Ag/63.5 g Cu
- From 20.0 g Cu, the following amount of Ag will be obtained:
20.0 g Cu x 215.8 g Ag/63.5 g Cu = 67.97 g Ag (theoretical amount)
- For an actual amount of 60.0 g of Ag, we calculate the percent yield as follows:
%yield = actual amount/theoretical amount x 100 =
= 60.0 g/67.97 g x 100 = 88.3 %
Who was the first person to propose the existence of the atom?.
Answers
Answer:
Democritus was Greek and was the first person to propose the idea that matter was not indefinitely divisible. He believed that matter was made up of individual particles called atomos. He believed that atoms could not be created, destroyed, or further divided.
Explanation:
quizlet
calculate the work in joules if 0.5 moles of al metal reacts with excess hydrochloric acid solution at 25 ° c and 1 atm (assume all gases behave ideally): 2al(s) 6hcl(aq) → 2alcl3(aq) 3h2(g)
a.1858.1j
b.1238.7j
c.1858.1j
d.1238.7j
Answers
The amount of work in joules when 0.5 moles of Al metal reacts with excess hydrochloric acid solution at 25 °C and 1 atm is c) 1858.1 J.
To calculate the work in joules, we can use the following formula for work done by an ideal gas at constant temperature and pressure:
W = -nRT
where W is the work done, n is the moles of gas, R is the ideal gas constant (8.314 J/mol·K), and T is the temperature in Kelvin.
From the balanced equation, 2 moles of Al produces 3 moles of H₂ gas. Since we have 0.5 moles of Al, the amount of H₂ gas produced will be:
(0.5 moles Al) * (3 moles H₂ / 2 moles Al) = 0.75 moles H₂
Now, we can plug in the values into the formula:
W = -(0.75 moles) * (8.314 J/mol·K) * (25 °C + 273.15 K)
W = -0.75 * 8.314 * 298.15 J
W = -1858.1 J
Therefore, the work done in joules is -1858.1 J (negative sign indicates that work is done by the system), which corresponds to option C.
learn more about work done here: https://brainly.com/question/27517477
#SPJ11
Select all the substances that require facilitated diffusion to cross a membrane. (you can select multiple)
A. negativity charged ions
B. hydrophilic molecules
C. molecules with no charge
D. hydrophilic molecules
Answers
A: Negatively charged ions
B: Hydrophollic molecules
Facilitated diffusion is the spontaneous passive transport of molecules across the biological membrane through the help of a membrane protein. This type of diffusion occurs in the molecules that cannot cross the membrane freely and requires a molecule to enter and leave the membrane.
Negatively charged ions and hydrophobic molecules require facilitated diffusion.
What occurs in facilitated diffusion?Channel and carrier proteins are needed for the facilitated diffusion because the molecules are not self-sufficient to cross the membrane on their own. Charged, polar ions and water-fearing molecules are transported with the help of certain protein channels and carriers that facilitates the molecules from the varied concentration environment.Therefore, option A and B are correct.
Learn more about facilitated diffusion here:
https://brainly.com/question/11240277
In the illustration, which solute will dissolve first?
00.0
00.0
tanka
tank
Solute in Tanks A and B will dissolve at equal rates.
Solute in Tonk Awill dissolve first
Soluten Tork B wil dissolves

Answers
The first to dissolve is the solute in tank B. The amount of a substance that dissolves in a specific solvent concentration at a specific temperature is known as its solubility.
Which phase of the dissolving process is the first?The introduction of a solute to a solvent is the first stage in the dissolving process. The molecules of these two substances begin to interact, causing the solute molecules to disperse and become encircled by solvent molecules.
Which of the following three compounds dissolves in water?In water, salt, sugar, and coffee all dissolve. They disintegrate quickly. They typically dissolve more rapidly and completely in hot or warm water. Even very hot water won't be able to dissolve sand or pepper.
To know more about temperature visit:
https://brainly.com/question/14633960
#SPJ1
What causes jet streams to form?
Answers
Answer:
Jet streams form when warm air masses meet cold air masses in the atmosphere. The Sun doesn't heat the whole Earth evenly. That's why areas near the equator are hot and areas near the poles are cold
If a car tire containing 5.61-L of gas at 29.68oC and 792.04-mmHg is driven high into the mountains where at altitude there is a pressure of 736.41-mmHg and the temperature is 7.35oC, its volume there is:
If a car tire containing 5.61-L of gas at 29.68oC and 792.04-mmHg is driven high into the mountains where at altitude there is a pressure of 736.41-mmHg and the temperature is 7.35oC, its volume there is:
Answers
There is a pressure of 736.41-mmHg and the temperature is 7.35oC, its volume there is 5.68 liters .
What is temperature ?The kinetic energy of atom-scale particles is essentially tied to temperature. If one glass of water is found to be hotter than another, it signifies that its water molecules have a larger average kinetic energy than the molecules in the colder glass: the higher the average kinetic energy of the particles, the higher the temperature
The Celsius temperature scale is utilized in the majority of scientific activity. The Celsius scale is based on the earlier centigrade scale, which has been somewhat modified to allow for the absolute temperature scale, which is measured in kelvins and denoted by the symbol K.
To know more about Temperature , visit ;
brainly.com/question/11464844
#SPJ1
What letter represents the trough? :) PLEASE FAST!
1) A
2) B
3) C
4) D
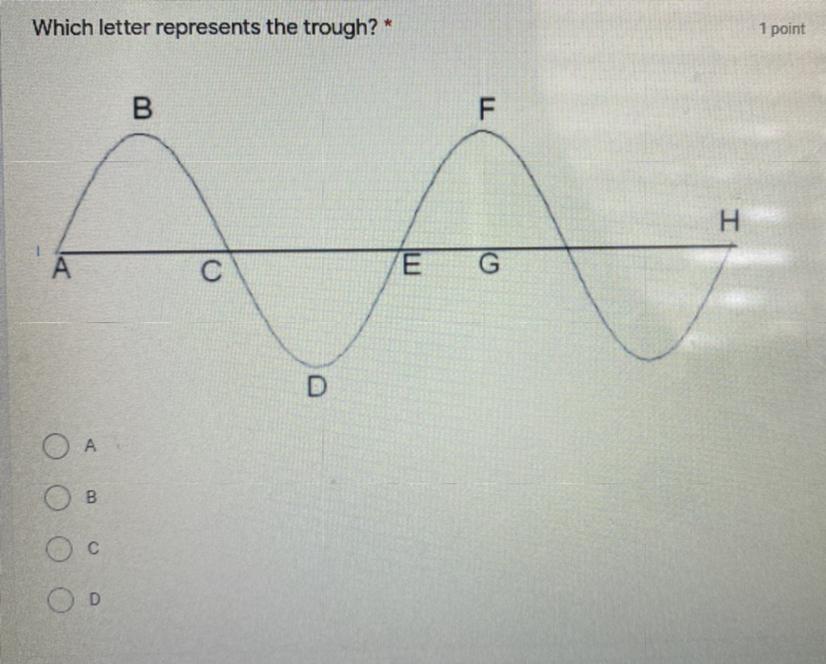
Answers
What is the difference in degrees Fahrenheit between the maximum expected temperatures by the end of the century between the lower and higher emissions scenario? Lower Emissions Scenario - Projected T
Answers
The difference in degrees Fahrenheit between the maximum expected temperatures by the end of the century between the lower and higher emissions scenarios can vary depending on various factors and assumptions.
However, in general, the lower emissions scenario is expected to result in a lower increase in global temperatures compared to the higher emissions scenario.
This means that the maximum expected temperature rise by the end of the century under the lower emissions scenario would be lower than that of the higher emissions scenario.
The specific temperature difference would depend on the specific projections and models used, but it highlights the significant impact that emissions reductions can have on mitigating future temperature increases.
To know more about global temperatures, refer here:
https://brainly.com/question/14367287#
#SPJ11
Mass is a(n)
property.
O a. electrical
Oc. chemical
Ob. physical
O d. natural
Answers
Answer:
d. natural I think is the right answer
Explanation:
I think thats the right answer
The equation for the complete combustion of ethene (C 2 H 4 ) is C 2 H 4 + 3O 2 2CO 2 + 2H 2 O If 2.70 mol of C 2 H 4 reacted with 6.30 mol , O 2 a. Identify the limiting reagent. b. Calculate the moles of water produced .
Answers
Answer:
22
Explanation:
Write a simple procedure for measuring out 2.6 grams of sodium bicarbonate NaHCO3 or commonly known as baking soda?
PLEASE I REALLY NEED THIS!!! :/
Answers
Answer:
See explanation
Explanation:
If I intend to measure 2.6 g of sodium bicarbonate, I need a spatula, a filter paper, and a weighing balance.
The filter paper is placed on the balance and the balance is adjusted to read zero. The spatula is now used to collect the sodium bicarbonate solid from its container and gradually drop it on the filter paper while watching the mass read out by the balance. This continues until the balance reads 2.6g.
PLEASE HELP IM DESPERATE.
Assume you are performing the calibration step of Experiment 8 and you begin with 80 g of water at 20 oC and 80 g of water at 80 oC. After adding the two portions of water into your calorimeter setup and following the procedure outlined in the experiment, you determine the temperature of the mixed portions of water to be 45 oC. What is the heat capacity of the calorimeter?
Assume room temperature is 25 oC.
Answers
Answer:
Reaction time tests can be used to assess an individual's hand-eye coordination. One test involves catching a 1 metre ruler and measuring the distance, d in metres, that the ruler travelled before being caught. The reaction time t seconds is then calculated using the formula: = √2d/9.8
how many electrons does a neutral atom of arsenic have
Answers
A neutral atom of arsenic has 33 electrons, balancing the 33 positively charged protons in its nucleus to maintain electrical neutrality.
The atomic number of arsenic (As) is 33, which means it has 33 protons in its nucleus. In a neutral atom, the number of electrons is equal to the number of protons. Therefore, a neutral atom of arsenic has 33 electrons.
Electrons are negatively charged particles that orbit the nucleus of an atom in specific energy levels or electron shells. The number of electrons determines the atom's chemical properties and its ability to form bonds with other atoms.
In the case of arsenic, the electron configuration can be determined by distributing the electrons into the available energy levels. The electron configuration of arsenic is\(1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^3.\)
This configuration indicates that there are 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, 6 electrons in the 2p orbital, 2 electrons in the 3s orbital, 6 electrons in the 3p orbital, 2 electrons in the 4s orbital, 10 electrons in the 3d orbital, and 3 electrons in the 4p orbital. Adding up these numbers gives a total of 33 electrons, which matches the atomic number of arsenic.
For more such questions on neutral atom visit:
https://brainly.com/question/24652228
#SPJ8
The molecular weight of table salt, NaCl, is 58. 5 g/mol. A tablespoon of salt weighs 6. 37 grams.
Calculate the number of moles of salt in one tablespoon.
First, complete the unit conversion using dimensional analysis:
Answers
=6.37g/58.5g/mol=0.109moles
:number of moles of salt in one tablespoon is 0.109moles
Answer:
Answer in picture
Explanation:

do activity for brainiest:)

Answers
Which transition metals have a maximum oxidation number of +4? a) Sc, Y, and La. b) Ti, Zr, and If. c) V, Nb, and Ta. d) Cr, Mo, and w. e) Mn, Tc, and Re.
Answers
The transition metals with a maximum oxidation number of +4 are found in Ti, Zr, and If. So, the correct answer is B.
Titanium (Ti) has oxidation states ranging from +2 to +4, with +4 being the most common and stable state. Zirconium (Zr) also has a maximum oxidation state of +4, which is the most stable and common for this element. However, there seems to be a typo in "If" - it should be Hf, which stands for Hafnium.
Hafnium (Hf) exhibits a maximum oxidation state of +4, and this state is the most prevalent for this element as well.
So, the correct answer is Ti, Zr, and Hf, which is option B.
Learn more about oxidation at https://brainly.com/question/31845824
#SPJ11
3. What is the definition of acceleration?
The change in velocity multiplied by the time of change
The change in speed
The change in velocity
The change in velocity divided by the time during which the change occurs

Answers
Answer:
D. The change in velocity divided by the time during which the change occurs.
Explanation: I took the quiz on Accelerate and it was correct
Answer:
D. The change in velocity divided by the time during which the change occurs.
Explanation:
Which is the BEST question to find out whether an object has the right property for you to carry it in your backpack?
What does the object sound like?
What does the object taste like?
What is the color of the object?
What is the size of the object?
Answers
Answer: what is the size of the object
Explanation: then again though, i wouldn't carry something in my backpack that didn't taste good...
1. What is the mass of 200 mL of ethanol if the density of ethanol is 0.789g/mL?
Answers
Answer:
The answer is 157.8 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question we have
mass = 0.789 × 200
We have the final answer as
157.8 gHope this helps you
In a titration, when the number of moles of hydrogen ions equals the number of moles of hydroxide ions, what is said to have happened?
The equivalence point has been reached.
The point of neutralization has been reached.
The end point has been reached.
Answers
Which of the following is not a benefit of using biofuels instead of fossil
fuels?
A. Biofuels produce less carbon dioxide when combusted.
B. Biofuels are more sustainable than fossil fuels.
C. Biofuels are renewable.
D. Fossil fuels may be used in the production of biofuels,
Answers
Answer: Fossil fuels may be used in the production of biofuels.
Explanation: Defeats the purpose of using biofuels over fossil fuels if one contains the other.
Answer:
D
Biofuel Disadvantages:
Labor costs are high, and storage space is limited. Excessive water use, particularly in dry areas. Increasing biomass for biofuel production raises agricultural land requirements.
Mark the statements which are correct. (Select all that apply. )
1 g = 10^3 mg
10^-3 g = 10^12 ng
1 s = 10^6 μs
1 km = 10^5 mm
1 s = 10^3 ms
Answers
All statements given in the question are incorrect except for 1 statement. The correct statement is:1 s = 10^3 ms.
In the question, we have been provided with 5 statements. We are asked to select all the correct statements from those 5 statements. Given below are for each statement:1 g = 10^3 mg:This is incorrect. 1 g is equal to 1000 mg.10^-3 g = 10^12 ng:This is incorrect. 10^-3 g is equal to 1 mg.1 km = 10^5 mm:This is incorrect. 1 km is equal to 1,000,000 mm.1 s = 10^6 μs:This is incorrect. 1 s is equal to 1,000,000 μs.1 s = 10^3 ms:This is correct. 1 s is equal to 1000 ms.Therefore, the main answer to this question is that only 1 statement is correct, which is:1 s = 10^3 ms.
Metric units are based on the power of ten. The base units of the International System of Units (SI) are the meter, kilogram, second, kelvin, ampere, mole, and candela. All other metric units can be derived from these basic units.The first unit in each conversion is in grams, seconds, or kilometers. The metric units for millimeters, microseconds, and nanograms are derived from these basic units. One gram is equal to 1000 milligrams (mg), 1 second is equal to 1000 milliseconds (ms), and 1 kilometer is equal to 1000000 millimeters (mm). 10^-3 g is equal to 1 milligram (mg), 10^6 μs is equal to 1 second (s), and 10^12 ng is equal to 1 gram (g).
To know more about except visit:
https://brainly.com/question/14400269
#SPJ11
Which statement correctly explains how matter is conserved in chemical reactions? (1 point)
A. The number of atoms in the reactants is always equal to the number of atoms in the products.
B. The states of matter of the reactants are always the same as the states of matter of the products.
C. The number of reactants is always equal to the number of products.
D. The number of molecules in the reactants is always equal to the number of molecules in the products.
Answers
According to the Law of Conservation of Matter, matter cannot be created nor destroyed. This means when a chemical process is underway, matter can’t be destroyed or created during the cycle. Balanced chemical equations, such as photosynthesis, prove the Law of Conservation of Matter to be correct because the same number of atoms were sustained on both sides of the equation.
This property of water helps make it the universal solvent: _____.
Responses
A solvencysolvency
B polarity
Answers
Answer: B: Polarity
Explanation:
Explanation: Water molecules have a polar arrangement of oxygen and hydrogen atoms—one side (hydrogen) has a positive electrical charge and the other side (oxygen) had a negative charge. This allows the water molecule to become attracted to many other different types of molecules.
Why do we standardize the naoh solution which we made by dissolving a measured mass of solid NaOH?
Answers
Standardizing a sodium hydroxide solution is necessary because NaOH reacts with atmospheric carbon dioxide to form sodium carbonate and water, reducing the accuracy of its concentration.
We standardize a sodium hydroxide (NaOH) solution, which means we determine its exact concentration, because NaOH is a strong base that reacts with atmospheric carbon dioxide (CO₂) to form sodium carbonate (Na₂CO₃) and water (H₂O):
2NaOH (aq) + CO₂ (g) → Na₂CO₃ (aq) + H₂O (l)
This reaction reduces the concentration of NaOH in the solution, making it less accurate for titrations or other chemical analyses. Standardizing the NaOH solution involves titrating it with a known concentration of an acid, such as hydrochloric acid (HCl), using an appropriate indicator to determine the exact concentration of NaOH.
During the titration, the acid and base react in a 1:1 ratio according to the balanced chemical equation:
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
The endpoint of the titration is reached when all the HCl has reacted with the NaOH, and the solution becomes neutral. An indicator, such as phenolphthalein, is used to signal the endpoint of the titration, where the indicator changes color from pink to colorless. The volume of acid required to reach the endpoint is measured, and the concentration of NaOH is calculated using stoichiometry.
Standardizing the NaOH solution ensures that its concentration is accurately known, allowing it to be used in subsequent chemical reactions or analyses. It is important to standardize the NaOH solution periodically, as the concentration can change over time due to factors such as atmospheric carbon dioxide absorption, water absorption, or contamination.
To know more about solution please refer: https://brainly.com/question/30665317
#SPJ4
A 2.89 g sample of phosphorus was burned in a large excess of chlorine, and the phosphorus chloride product was found to have a mass of 12.87 g . The vapor of this phosphorus chloride effused at a rate that was 1.77 times slower than that of the same amount of CO2 at the same temperature and pressure. Determine the molar mass and the molecular formula of this phosphorus chloride.
Answers
The molar mass and the molecular formula of the phosphorus chloride is 214.01 g/mol and (PCl2), respectively.
The given mass of phosphorus chloride is 12.87 g. Thus, its molecular mass can be calculated as follows:
Molecular mass of phosphorus chloride = (mass of phosphorus chloride)/(number of moles of phosphorus chloride)
Thus, the number of moles of phosphorus chloride can be calculated as follows:
Number of moles of phosphorus chloride = (mass of phosphorus chloride)/(molar mass of phosphorus chloride)
The molar mass of phosphorus chloride can be calculated as follows:
Molar mass of phosphorus chloride = Molar mass of P + Molar mass of Cl
= 30.97 g/mol + 2(35.45 g/mol)
= 101.87 g/mol
Next, the rate of effusion of phosphorus chloride is 1.77 times slower than that of CO2 at the same temperature and pressure.
The rate of effusion of a gas is inversely proportional to the square root of its molar mass. Thus, we have:
Rate of effusion of phosphorus chloride/Rate of effusion of CO2
= (Molar mass of CO2/Molar mass of phosphorus chloride)1/2
Substituting the given values, we get:
1/1.77 = (44.01 g/mol/Molar mass of phosphorus chloride)1/2
On solving, we get:
Molar mass of phosphorus chloride = 214.01 g/mol
Now, the molecular formula of the phosphorus chloride can be determined by dividing its molar mass by its empirical formula mass and multiplying the resulting value by the empirical formula:
Empirical formula mass of phosphorus chloride = 30.97 g/mol + 2(35.45 g/mol)
= 101.87 g/mol
Thus, the value of n in the molecular formula (PClx)
n can be calculated as follows:
n = Molar mass of phosphorus chloride/Empirical formula mass of phosphorus chloride
= 214.01 g/mol/101.87 g/mol
= 2.10 approx.
Rounding off to the nearest whole number, we get:
n = 2
Thus, the molecular formula of the phosphorus chloride is (PCl2).
Therefore, the molar mass and the molecular formula of the phosphorus chloride is 214.01 g/mol and (PCl2), respectively.
To know more about molar visit:
https://brainly.com/question/31545539
#SPJ11
Write the equilibrium constant expression for each of the following reactions.
a CO(g) + H2O(g) YZZ ZZX CO2(g) + H2(g)
b CH4(g) + Cl2(g) YZZ ZZX CH3 Cl(g) + HCl(g)
c 2SO2(g) + O2(g) YZZ ZZX 2SO3(g)
d FeO(s) + CO(g) YZZ ZZX Fe(s) + CO2(g)
Answers
The equilibrium constant expression for each of the reaction are K = [\(CO_2\)][\(H_2\)] / [CO][\(H_2O\)].
The equilibrium constant expression for each of the reactions you provided can be written as follows:
a) CO(g) + \(H_2O\)(g) ⇄ \(CO_2\)(g) + \(H_2\)(g)
The equilibrium constant expression for this reaction is:
K = [\(CO_2\)][\(H_2\)] / [CO][\(H_2O\)].
b) \(CH_4\)(g) + \(Cl_2\)(g) ⇄ \(CH_3Cl\)(g) + HCl(g)
The equilibrium constant expression for this reaction is:
\(K = [CH_3Cl][HCl] / [CH_4][Cl_2]\)
c) \(2SO_2\)(g) + \(O_2\)(g) ⇄ \(2SO_3\)(g)
The equilibrium constant expression for this reaction is:
\(K = [SO_3]^2 / [SO_2]^2[O_2]\)
d) FeO(s) + CO(g) ⇄ Fe(s) + \(CO_2\)(g)
The equilibrium constant expression for this reaction is:
K = [\(CO_2\)][Fe] / [CO]
In each expression, the brackets [] denote the concentration of the respective species at equilibrium.
Thus, the equilibrium constant (K) is a dimensionless quantity that represents the ratio of the concentrations of the products to the concentrations of the reactants, with each concentration term raised to the power corresponding to its stoichiometric coefficient in the balanced equation.
For more details regarding equilibrium constant, visit:
https://brainly.com/question/28559466
#SPJ1
Topic: Mass Balance. A company sells fishmeal to be used as a protein supplement in certain foods. The process consists of: a. Extraction of fish oil, stage in which a pasta is obtained that has 20% flour and 80% water. b. Drying of pasta in a rotary drum, which produces fishmeal with 40% humidity. How much pasta must be input to the process to produce 1000 kg ?
Answers
To produce 1000 kg of fishmeal (M = 1000 kg), you would need 3000 kg of pasta. To determine the amount of pasta required to produce 1000 kg of fishmeal, we need to consider the mass balance of the process. Let's break down the steps involved:
A. Extraction of fish oil:
The pasta obtained from the extraction stage contains 20% flour and 80% water. To calculate the amount of pasta, we need to determine the mass of flour and water in the pasta. Let's assume the total mass of the pasta is P kg.
Mass of flour = 20% of P = 0.2P kg
Mass of water = 80% of P = 0.8P kg
b. Drying of pasta:
During the drying stage, the pasta is dried in a rotary drum, resulting in fishmeal with 40% humidity. This means that the final fishmeal will contain 60% dry matter.
Let's assume the mass of the dried fishmeal is M kg.
Mass of dry matter = 60% of M = 0.6M kg
Since the dry matter in the fishmeal comes from the flour in the pasta, we can equate the mass of dry matter to the mass of flour:
0.6M kg = 0.2P kg
To produce 1000 kg of fishmeal, we want to find the corresponding value of P:
0.6M = 0.2P
P = (0.6M) / 0.2
P = 3M
Therefore, to produce 1000 kg of fishmeal (M = 1000 kg), you would need 3000 kg of pasta.
To know more about mass balance, click here, https://brainly.com/question/17014679
#SPJ11
predict if a reaction would occur when solutions of 0.1 m naoh and 0.1 m kcl are combined. if you predict a reaction will occur, determine the net ionic equation for the reaction.
Answers
There are no H⁺ ions present in the reaction, no acid-base neutralization reaction occurs when 0.1 M NaOH and 0.1 M KCl solutions are combined. Therefore, we cannot predict a reaction occurring between these solutions.
To determine if a reaction would occur when 0.1 M NaOH and 0.1 M KCl solutions are combined, we need to look at the possible chemical reactions that could occur. NaOH is a strong base and KCl is a salt, so we could potentially see an acid-base neutralization reaction between the NaOH and KCl. The balanced equation for this reaction would be:
NaOH + KCl ⇒ NaCl + KOH
To determine the net ionic equation, we need to write the balanced equation in ionic form and then cancel out the spectator ions (ions that appear on both sides of the equation and do not participate in the reaction). The net ionic equation for the reaction above is:
Na+(aq) + OH⁻(aq) + K⁺(aq) + Cl⁻(aq) ⇒ Na⁺(aq) + Cl⁻(aq) + K⁺(aq) + OH⁻(aq)
After canceling out the spectator ions, we are left with the net ionic equation:
OH⁻(aq) + H⁺(aq) ⇒ H₂O(l)
Because they are not involved in the process, the repeating ions need to be taken out of the equation.
Learn more about net ionic equation here
https://brainly.com/question/15466794
#SPJ11